Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0E1WI
|
|||
| Drug Name |
Fentanyl
|
|||
| Synonyms |
fentanyl; Fentanil; Phentanyl; Fentora; Sublimaze; Fentanila; Fentanest; Duragesic; Fentanylum; Durogesic; Sentonil; 437-38-7; Duragesic-100; IONSYS; Duragesic-25; Duragesic-75; Duragesic-50; Duragesic-12; Fentanyl-25; Fentanyl-75; Fentanyl-12; Fentanyl-50; Fentanylum [INN-Latin]; Fentanila [INN-Spanish]; Fentanyl-100; 1-Phenethyl-4-N-propionylanilinopiperidine; Matrifen; Sublimase; N-(1-Phenethylpiperidin-4-yl)-N-phenylpropionamide; N-(1-Phenethyl-4-piperidyl)propionanilide; N-Phenethyl-4-(N-propionylanilino)piperidine; Fentanil [DCIT]; Subsys; Fentanyl-37; Fentanyl-62; Fentanyl-87; Abstral; Actiq; Lazanda; Onsolis; Fentanyl Citrate; Fentanyl Citrate Preservative Free; Sublimaze Preservative Free; fentanyl (transmucosal film, pain), Auxilium Pharmaceuticals
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Analgesia [ICD-11: MB40.8; ICD-10: R20.0; ICD-9: 338] | Approved | [1] | |
| Cancer related pain [ICD-11: MG30; ICD-10: R52.1, R52.2] | Phase 3 | [2] | ||
| Pain [ICD-11: MG30-MG3Z] | Phase 3 | [3] | ||
| Chronic pain [ICD-11: MG30; ICD-10: R52.1, R52.2; ICD-9: 338.2, 780] | Phase 1 | [4] | ||
| Therapeutic Class |
Neurology Agents
|
|||
| Company |
Janssen Pharmaceuticals Inc
|
|||
| Structure |
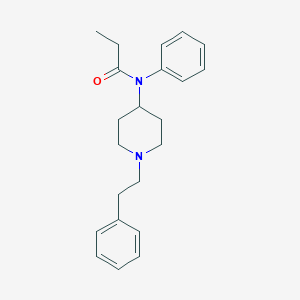 |
Download2D MOL |
||
| Formula |
C22H28N2O
|
|||
| Canonical SMILES |
CCC(=O)N(C1CCN(CC1)CCC2=CC=CC=C2)C3=CC=CC=C3
|
|||
| InChI |
1S/C22H28N2O/c1-2-22(25)24(20-11-7-4-8-12-20)21-14-17-23(18-15-21)16-13-19-9-5-3-6-10-19/h3-12,21H,2,13-18H2,1H3
|
|||
| InChIKey |
PJMPHNIQZUBGLI-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 437-38-7
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:119915
|
|||
| ADReCS Drug ID | BADD_D00879 ; BADD_D00880 ; BADD_D00881 | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Opioid receptor mu (MOP) | Target Info | Modulator | [5], [6] |
| KEGG Pathway | Neuroactive ligand-receptor interaction | |||
| Estrogen signaling pathway | ||||
| Morphine addiction | ||||
| NetPath Pathway | TCR Signaling Pathway | |||
| Panther Pathway | Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | |||
| Heterotrimeric G-protein signaling pathway-Gq alpha and Go alpha mediated pathway | ||||
| Enkephalin release | ||||
| Pathway Interaction Database | IL4-mediated signaling events | |||
| Reactome | Peptide ligand-binding receptors | |||
| G alpha (i) signalling events | ||||
| WikiPathways | TCR Signaling Pathway | |||
| GPCRs, Class A Rhodopsin-like | ||||
| Peptide GPCRs | ||||
| Opioid Signalling | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | ClinicalTrials.gov (NCT00822614) Safety of Fentanyl TAIFUN Treatment. U.S. National Institutes of Health. | |||
| REF 3 | A Phase III study to assess the clinical utility of low-dose fentanyl transdermal system in patients with chronic nonmalignant pain. Curr Med Res Opin. 2006 Aug;22(8):1493-501. | |||
| REF 4 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 5 | The ChEMBL database in 2017. Nucleic Acids Res. 2017 Jan 4;45(D1):D945-D954. | |||
| REF 6 | Synthesis and pharmacological studies of new hybrid derivatives of fentanyl active at the mu-opioid receptor and I2-imidazoline binding sites.Bioorg Med Chem.2006 Oct 1;14(19):6570-80. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

