Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0E3ZC
|
|||
| Drug Name |
Oseltamivir
|
|||
| Synonyms |
Tamiflu (*Phosphate salt 1:1*); Agucort; Oseltamivirum; Tamvir; GS 4104; GS4104; Agucort (TN); GS-4104; Oseltamivir (INN); Oseltamivir [INN:BAN]; Ro-640796; Tamiflu (TN); Tamiflu-Free; Ro-64-0796; OTV
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Influenza virus infection [ICD-11: 1E30-1E32] | Approved | [1] | |
| Coronavirus Disease 2019 (COVID-19) [ICD-11: 1D6Y] | Phase 3 | [2] | ||
| Therapeutic Class |
Antiviral Agents
|
|||
| Company |
Hoffmann-La Roche pharmaceutical company
|
|||
| Structure |
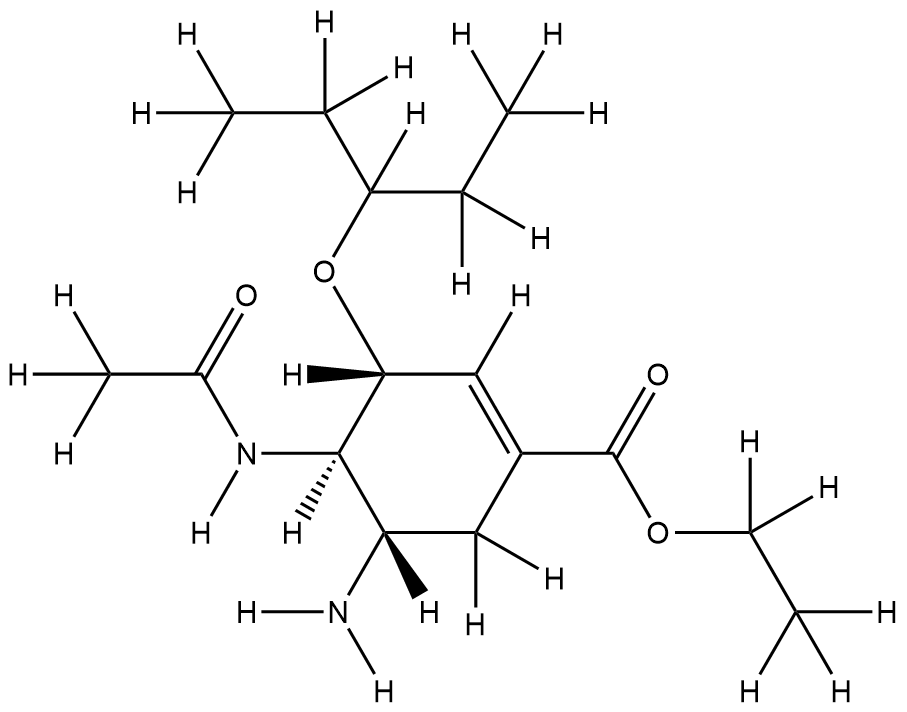 |
Download2D MOL |
||
| Formula |
C16H28N2O4
|
|||
| Canonical SMILES |
CCC(CC)OC1C=C(CC(C1NC(=O)C)N)C(=O)OCC
|
|||
| InChI |
1S/C16H28N2O4/c1-5-12(6-2)22-14-9-11(16(20)21-7-3)8-13(17)15(14)18-10(4)19/h9,12-15H,5-8,17H2,1-4H3,(H,18,19)/t13-,14+,15+/m0/s1
|
|||
| InChIKey |
VSZGPKBBMSAYNT-RRFJBIMHSA-N
|
|||
| CAS Number |
CAS 196618-13-0
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
10292, 626306, 7980209, 8034664, 8189453, 14825762, 14898934, 43121870, 46507602, 49958481, 50123365, 53789667, 57315247, 58106749, 75193389, 85789649, 92309047, 93166584, 96024993, 103375662, 104332669, 117576917, 118048381, 124893596, 126657793, 126681234, 126733879, 134338002, 135023115, 135811666, 137002940, 137236650, 140589553, 151982861, 152099920, 152258534, 160647368, 160963546, 164786722, 164831566, 165702338, 174006818, 175267600, 176484257, 179116828, 184546341, 185976796, 196109901, 211535901, 223556618
|
|||
| ChEBI ID |
CHEBI:7798
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | ClinicalTrials.gov (NCT04261270) A Randomized,Open,Controlled Clinical Study to Evaluate the Efficacy of ASC09F and Ritonavir for 2019-nCoV Pneumonia | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

