Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0E4DW
|
|||
| Former ID |
DAP000130
|
|||
| Drug Name |
Phenytoin
|
|||
| Synonyms |
Aleviatin; Antisacer; Auranile; Causoin; Citrullamon; Citrulliamon; Comital; Comitoina; Convul; Danten; Dantinal; Dantoinal; Dantoine; Denyl; Difenilhidantoina; Difenin; Difetoin; Difhydan; Dihycon; Dihydantoin; Dilabid; Dilantin; Dilantine; Dillantin; Dintoin; Dintoina; Diphantoin; Diphedal; Diphedan; Diphenat; Diphenin; Diphenine; Diphentoin; Diphentyn; Diphenylan; Diphenylhydantoin; Diphenylhydantoine; Diphenylhydatanoin; Ditoinate; Ekko; Elepsindon; Enkelfel; Epamin; Epanutin; Epelin; Epifenyl; Epihydan; Epilantin; Epinat; Epised; Eptal; Eptoin; Fenitoin; Fenitoina; Fentoin; Fenylepsin; Fenytoine; Hidan; Hidantal; Hidantilo; Hidantina; Hidantomin; Hindatal; Hydantal; Hydantin; Hydantoinal; Hydantol; Idantoil; Idantoin; Iphenylhydantoin; Kessodanten; Labopal; Lehydan; Lepitoin; Lepsin; Minetoin; Neosidantoina; Novantoina; Novophenytoin; Oxylan; PHENYTEK; Phanantin; Phanatine; Phenatine; Phenatoine; Phenhydan; Phenhydanin; Phenitoin; Phentoin; Phentytoin; Phenytex; Phenytoine; Phenytoinum; Ritmenal; Saceril; Sanepil; Silantin; Sinergina; Sodanthon; Sodantoin; Sodanton; Solantin; Solantoin; Solantyl; Sylantoic;TOIN; Tacosal; Thilophenyl; Zentronal; Zentropil; Component of Mebroin; Dantoinal klinos; Difenilhidantoina [Spanish]; Dihydan toin; Dilantin acid; Diphenylhydantoine [French]; Ekko capsules; Elepsi ndon; Epdantoin Simple; Epdantoine simple; Epilan D; Extended Phenytoin Sodium; Fen toin; Fenantoin Mn Pharma; Fenidantoin s; Fenytoin Dak; Hidantina senosian; Hidantina vitoria; Ictalis simp le; Ictalis simple; Om hidantoina simple; Phenat ine; Phenytoin AWD; Sodium Diphenylhydantoinate; Taco sal; Toin unicelles; CL12003; D 4007; D4007_SIGMA; Didan TDC 250; Epasmir 5; IFLab1_000214; DILANTIN-30; DPH (VAN); Di-Hydan; Di-Lan; Di-Phetine; Dilantin (TN); Dilantin Infatabs (TN); Dilantin Kapseals (TN); Dilantin-125; Diphenylhydantoin (VAN); Epanutin (TN); Epasmir (5); Epilan-D; Eptoin (TN); Fenidantoin (s); Fenitoina [INN-Spanish]; Neos-Hidantoina; Om-Hydantoine; PHENYTOIN SODIUM, EXTENDED; Phenytek (TN); Phenytoin (PHN); Phenytoin-Gerot; Phenytoine [INN-French]; Phenytoinum [INN-Latin]; Di-Lan (VAN); Didan-tdc-250; Gerot-epilan-D; PHENYTOIN (5,5-DIPHENYLHYDANTOIN); Phenytoin (JP15/USP/INN); Phenytoin [USAN:INN:BAN:JAN]; Hydantoin, 5,5-diphenyl-(8CI); 2,4-Imidazolidinedione, 5,5-diphenyl-(9CI); 2-hydroxy-5,5-diphenyl-3,5-dihydro-4H-imidazol-4-one; 5,5-DIPHENYLHYDANTOIN; 5,5-Diphenyl-2,4-imidazolidinedione; 5,5-Diphenyl-imidazolidine-2,4-dione; 5,5-Diphenylhydantoin (IUPAC); 5,5-Diphenylhydantoin (phenytoin); 5,5-Diphenylimidazolidin-2,4-dione; 5,5-Dwufenylohydantoina; 5,5-Dwufenylohydantoina [Polish]; 5,5-diphenylimidazolidine-2,4-dione
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Epilepsy [ICD-11: 8A60-8A68] | Approved | [1] | |
| Therapeutic Class |
Anticonvulsants
|
|||
| Company |
Mylan Laboratories
|
|||
| Structure |
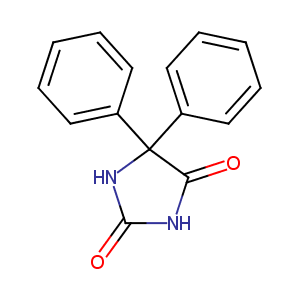 |
Download2D MOL |
||
| Formula |
C15H12N2O2
|
|||
| Canonical SMILES |
C1=CC=C(C=C1)C2(C(=O)NC(=O)N2)C3=CC=CC=C3
|
|||
| InChI |
1S/C15H12N2O2/c18-13-15(17-14(19)16-13,11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10H,(H2,16,17,18,19)
|
|||
| InChIKey |
CXOFVDLJLONNDW-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 57-41-0
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9647, 74340, 592179, 596082, 841957, 3136997, 4284342, 5171921, 7847578, 7980312, 8151275, 10527886, 10589150, 11111069, 11335682, 11353793, 11360921, 11363999, 11366561, 11369123, 11371880, 11374526, 11377285, 11461893, 11485017, 11489097, 11490706, 11492799, 11494919, 11532994, 14847761, 17389903, 17404941, 24278370, 26068808, 26753026, 26753027, 26753028, 29220975, 46508847, 47588955, 47736431, 47736432, 47795039, 48168464, 48184955, 48243361, 48413692, 48416427, 48422573
|
|||
| ChEBI ID |
CHEBI:8107
|
|||
| ADReCS Drug ID | BADD_D01765 ; BADD_D01766 | |||
| SuperDrug ATC ID |
N03AB02
|
|||
| SuperDrug CAS ID |
cas=000057410
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Metabolism of Drug Affected by Studied Microbe(s) | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Bacteroidales | ||||
|
Studied Microbe: Bacteroides dorei DSM 17855
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Experimental Method | High-throughput screening | |||
| Description | Phenytoin sodium can be metabolized by Bacteroides dorei DSM 17855 (log2FC = -0.618; p = 0.022). | |||
|
Studied Microbe: Bacteroides uniformis ATCC 8492
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Experimental Method | High-throughput screening | |||
| Description | Phenytoin sodium can be metabolized by Bacteroides uniformis ATCC 8492 (log2FC = -0.48; p = 0.021). | |||
|
Studied Microbe: Bacteroides vulgatus ATCC 8482
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Experimental Method | High-throughput screening | |||
| Description | Phenytoin sodium can be metabolized by Bacteroides vulgatus ATCC 8482 (log2FC = -0.555; p = 0.027). | |||
|
Studied Microbe: Bacteroides xylanisolvens DSM18836
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Experimental Method | High-throughput screening | |||
| Description | Phenytoin sodium can be metabolized by Bacteroides xylanisolvens DSM18836 (log2FC = -0.326; p = 0.002). | |||
|
Studied Microbe: Parabacteroides distasonis ATCC 8503
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Experimental Method | High-throughput screening | |||
| Description | Phenytoin sodium can be metabolized by Parabacteroides distasonis ATCC 8503 (log2FC = -0.364; p = 0.02). | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Sodium channel unspecific (NaC) | Target Info | Blocker | [3] |
| KEGG Pathway | Dopaminergic synapse | |||
| Reactome | Interaction between L1 and Ankyrins | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature. 2019 Jun;570(7762):462-467. | |||
| REF 3 | Lacosamide: a new approach to target voltage-gated sodium currents in epileptic disorders. CNS Drugs. 2009;23(7):555-68. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

