Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0F1CE
|
|||
| Former ID |
DNCL001817
|
|||
| Drug Name |
LCQ908
|
|||
| Synonyms |
Pradigastat; LCQ-908; 956136-95-1; UNII-2U23G6VNUZ; LCQ908; 2U23G6VNUZ; LCQ 908; Pradigastat [USAN:INN]; LCQ 908NXA; 2-[4-[4-[5-[[6-(trifluoromethyl)pyridin-3-yl]amino]pyridin-2-yl]phenyl]cyclohexyl]acetic acid; 2-{4-[4-(5-{[6-(trifluoromethyl)pyridin-3-yl]amino}pyridin-2-yl)phenyl]cyclohexyl}acetic acid; Pradigastat (USAN); LCQ908-NXA; SCHEMBL180536; GTPL7830; SCHEMBL1289309; CHEMBL2364624; SCHEMBL18286769; SCHEMBL16104874; GXALXAKNHIROPE-UHFFFAOYSA-N; BCP21089; ZINC253387875; AKOS027338695; DB12866; SB16971; CS-1222
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Familial chylomicronemia syndrome [ICD-11: 5C80; ICD-10: E78.4, E78.5] | Phase 3 | [1], [2] | |
| Hepatitis C virus infection [ICD-11: 1E51.1; ICD-10: B18.2] | Phase 3 | [1], [2] | ||
| Hypertriglyceridemia [ICD-11: 5C80.1; ICD-10: E78.1, E78.3; ICD-9: 272.1, 427] | Phase 3 | [3] | ||
| Company |
Novartis
|
|||
| Structure |
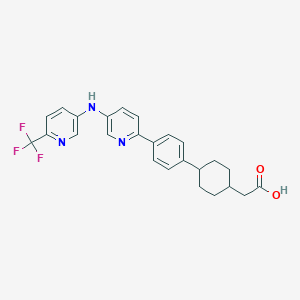 |
Download2D MOL |
||
| Formula |
C25H24F3N3O2
|
|||
| Canonical SMILES |
C1CC(CCC1CC(=O)O)C2=CC=C(C=C2)C3=NC=C(C=C3)NC4=CN=C(C=C4)C(F)(F)F
|
|||
| InChI |
1S/C25H24F3N3O2/c26-25(27,28)23-12-10-21(15-30-23)31-20-9-11-22(29-14-20)19-7-5-18(6-8-19)17-3-1-16(2-4-17)13-24(32)33/h5-12,14-17,31H,1-4,13H2,(H,32,33)
|
|||
| InChIKey |
GXALXAKNHIROPE-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 956136-95-1
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Diacylglycerol acyltransferase 1 (DGAT1) | Target Info | Modulator | [4], [5] |
| BioCyc | Triacylglycerol biosynthesis | |||
| KEGG Pathway | Glycerolipid metabolism | |||
| Retinol metabolism | ||||
| Metabolic pathways | ||||
| Fat digestion and absorption | ||||
| Pathwhiz Pathway | Retinol Metabolism | |||
| Reactome | Acyl chain remodeling of DAG and TAG | |||
| Triglyceride Biosynthesis | ||||
| WikiPathways | Vitamin A and Carotenoid Metabolism | |||
| Statin Pathway | ||||
| Triacylglyceride Synthesis | ||||
| Glycerophospholipid biosynthesis | ||||
| Fatty acid, triacylglycerol, and ketone body metabolism | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7830). | |||
| REF 2 | ClinicalTrials.gov (NCT01514461) A Randomized, Double-blind, Placebo Controlled Study to Assess Efficacy, Safety and Tolerability of LCQ908 in Subjects With Familial Chylomicronemia Syndrome. U.S. National Institutes of Health. | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | DGAT1 inhibitors as anti-obesity and anti-diabetic agents. Curr Opin Drug Discov Devel. 2010 Jul;13(4):489-96. | |||
| REF 5 | Acyltransferase inhibitors: a patent review (2010-present).Expert Opin Ther Pat. 2015 Feb;25(2):145-58. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

