Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0F2JY
|
|||
| Former ID |
DIB016972
|
|||
| Drug Name |
LY-2334737
|
|||
| Synonyms |
Gem prodrug (cancer), Eli Lilly; Gemcitabine prodrug (cancer), Lilly
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C00-D48; ICD-9: 140-199, 210-229] | Phase 1 | [1] | |
| Company |
Eli Lilly & Co
|
|||
| Structure |
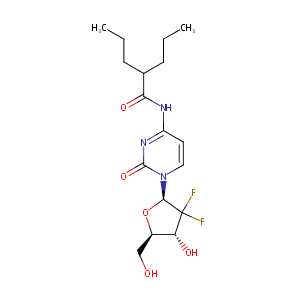 |
Download2D MOL |
||
| Formula |
C17H25F2N3O5
|
|||
| Canonical SMILES |
CCCC(CCC)C(=O)NC1=NC(=O)N(C=C1)C2C(C(C(O2)CO)O)(F)F
|
|||
| InChI |
1S/C17H25F2N3O5/c1-3-5-10(6-4-2)14(25)20-12-7-8-22(16(26)21-12)15-17(18,19)13(24)11(9-23)27-15/h7-8,10-11,13,15,23-24H,3-6,9H2,1-2H3,(H,20,21,25,26)/t11-,13-,15-/m1/s1
|
|||
| InChIKey |
MEOYFIHNRBNEPI-UXIGCNINSA-N
|
|||
| CAS Number |
CAS 892128-60-8
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT01648764) A Study of LY2334737 in Participants With Cancer That is Advanced and/or Has Spread. U.S. National Institutes of Health. | |||
| REF 2 | Phase I study of oral gemcitabine prodrug (LY2334737) in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2013 Jun;71(6):1645-55. | |||
| REF 3 | Role of gemcitabine in cancer therapy.Future Oncol.2005 Feb;1(1):7-17. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

