Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0F2WP
|
|||
| Former ID |
DCL000650
|
|||
| Drug Name |
Relugolix
|
|||
| Synonyms |
737789-87-6; TAK-385; TAK 385; UNII-P76B05O5V6; CHEMBL1800159; TAK-385/TAK385; P76B05O5V6; 1-(4-(1-(2,6-difluorobenzyl)-5-((dimethylamino)methyl)-3-(6-methoxypyridazin-3-yl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl)phenyl)-3-methoxyurea; Relugolix [USAN:INN]; TAK385; Relugolix (JAN/INN); SCHEMBL778416; GTPL5586; DTXSID40224167; MolPort-044-567-649; AOMXMOCNKJTRQP-UHFFFAOYSA-N; EX-A1083; BCP21587; ZINC43206033; BDBM50347982; AKOS027440398; SB16721; DB11853; CS-5917
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Prostate cancer [ICD-11: 2C82.0; ICD-9: 185] | Approved | [1] | |
| Endometriosis [ICD-11: GA10; ICD-10: N80, N80.9] | Phase 3 | [2] | ||
| Company |
Takeda
|
|||
| Structure |
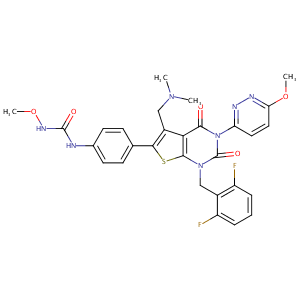 |
Download2D MOL |
||
| Formula |
C29H27F2N7O5S
|
|||
| Canonical SMILES |
CN(C)CC1=C(SC2=C1C(=O)N(C(=O)N2CC3=C(C=CC=C3F)F)C4=NN=C(C=C4)OC)C5=CC=C(C=C5)NC(=O)NOC
|
|||
| InChI |
1S/C29H27F2N7O5S/c1-36(2)14-19-24-26(39)38(22-12-13-23(42-3)34-33-22)29(41)37(15-18-20(30)6-5-7-21(18)31)27(24)44-25(19)16-8-10-17(11-9-16)32-28(40)35-43-4/h5-13H,14-15H2,1-4H3,(H2,32,35,40)
|
|||
| InChIKey |
AOMXMOCNKJTRQP-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 737789-87-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Gonadotropin-releasing hormone receptor (GNRHR) | Target Info | Antagonist | [1] |
| KEGG Pathway | Neuroactive ligand-receptor interaction | |||
| GnRH signaling pathway | ||||
| NetPath Pathway | IL1 Signaling Pathway | |||
| IL2 Signaling Pathway | ||||
| Panther Pathway | Heterotrimeric G-protein signaling pathway-Gq alpha and Go alpha mediated pathway | |||
| Reactome | Hormone ligand-binding receptors | |||
| G alpha (q) signalling events | ||||
| WikiPathways | Gastrin-CREB signalling pathway via PKC and MAPK | |||
| Peptide GPCRs | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| GPCRs, Other | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2020 | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

