Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0FJ9I
|
|||
| Drug Name |
Cotellic
|
|||
| Synonyms |
Cobimetinib fumarate; Cobimetinib Fumarate; Cobimetinib hemifumarate; Xl-518 hemifumarate; GDC-0973 hemifumarate; UNII-6EXI96H8SV; 6EXI96H8SV; Cobimetinib fumarate [USAN]; 1369665-02-0; Cobimetinib fumarate (USAN); Cotellic (TN); CHEMBL2364607; CHEBI:90853; Methanone, (3,4-difluoro-2-((2-fluoro-4-iodophenyl)amino)phenyl)(3-hydroxy-3-(2S)-2-piperidinyl-1-azetidinyl)-, (2E)-2-butenedioate (2:1); D10615; bis[(2S)-2-{1-[3,4-difluoro-2-(2-fluoro-4-iodoanilino)benzoyl]-3-hydroxyazetidin-3-yl}piperidin-1-ium] (2E)-but-2-enedioate
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Melanoma [ICD-11: 2C30; ICD-9: 172] | Phase 3 | [1] | |
| Breast cancer [ICD-11: 2C60-2C65] | Phase 2 | [2] | ||
| Head and neck cancer [ICD-11: 2D42] | Phase 1 | [2] | ||
| Renal cell carcinoma [ICD-11: 2C90; ICD-10: C64; ICD-9: 189] | Phase 1 | [2] | ||
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C00-D48; ICD-9: 140-199, 210-229] | Phase 1 | [3] | ||
| Urothelial carcinoma [ICD-11: 2C92.0] | Phase 1 | [2] | ||
| Company |
Genentech South San Francisco, CA
|
|||
| Structure |
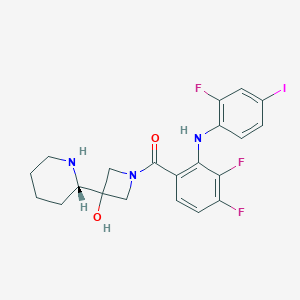 |
Download2D MOL |
||
| Formula |
C21H21F3IN3O2
|
|||
| Canonical SMILES |
C1CCNC(C1)C2(CN(C2)C(=O)C3=C(C(=C(C=C3)F)F)NC4=C(C=C(C=C4)I)F)O
|
|||
| InChI |
1S/C21H21F3IN3O2/c22-14-6-5-13(19(18(14)24)27-16-7-4-12(25)9-15(16)23)20(29)28-10-21(30,11-28)17-3-1-2-8-26-17/h4-7,9,17,26-27,30H,1-3,8,10-11H2/t17-/m0/s1
|
|||
| InChIKey |
BSMCAPRUBJMWDF-KRWDZBQOSA-N
|
|||
| CAS Number |
CAS 934660-93-2
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:90851
|
|||
| ADReCS Drug ID | BADD_D00513 | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | ClinicalTrials.gov (NCT01106599) A Study Evaluating the Safety, Tolerability, and Pharmacokinetics of GDC-0623 in Patients With Locally Advanced or Metastatic Solid Tumors. U.S. National Institutes of Health. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

