Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0FP0R
|
|||
| Former ID |
DCL000437
|
|||
| Drug Name |
Apaziquone
|
|||
| Synonyms |
EOquin; 114560-48-4; Apaziquonum; NOR-701; EO 9 (pharmaceutical); EO-9; Apaziquone [USAN:INN]; NSC-382459; Apaziquonum [INN-Latin]; E 09; NSC 382459; UNII-H464ZO600O; E-85/053; E-09; EO9; NSC 382456; H464ZO600O; 5-(Azridin-1-yl)-3-(hydroxymethyl)-2-((1E)-3-hydroxyprop-1-enyl)-methyl-1H-indole-4,7-dione; (E)-5-(1-Azirinyl)-3-(hydroxymethyl)-2-(3-hydroxy-1-propenyl)-1-methyl-1H-indole-4,7-dione; E09; 1H-Indole-4,7-dione, 5-(1-aziridinyl)-3-(hydroxymethyl)-2-(3-hydroxy-1-propenyl)-1-methyl-, (E)-; Neoquin; Qapzola; Apaziquonum; EO 9; Eoquin (TN); Apaziquone (USAN/INN); E-85/050; 3-hydroxymethyl-5-aziridinyl-1-methyl-2-(1H-indole-4,7-dione)prop-beta-en-alpha-ol; 5-(aziridin-1-yl)-3-(hydroxymethyl)-2-[(E)-3-hydroxyprop-1-enyl]-1-methylindole-4,7-dione; Apaziquone/EOquin
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Bladder cancer [ICD-11: 2C94; ICD-9: 188] | Phase 3 | [1] | |
| Non-muscle invasive bladder cancer [ICD-11: 2C94; ICD-10: C67, C67.9; ICD-9: 188] | Phase 3 | [2] | ||
| Company |
Spectrum
|
|||
| Structure |
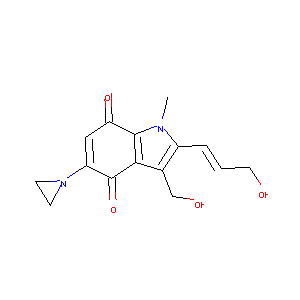 |
Download2D MOL |
||
| Formula |
C15H16N2O4
|
|||
| Canonical SMILES |
CN1C(=C(C2=C1C(=O)C=C(C2=O)N3CC3)CO)C=CCO
|
|||
| InChI |
1S/C15H16N2O4/c1-16-10(3-2-6-18)9(8-19)13-14(16)12(20)7-11(15(13)21)17-4-5-17/h2-3,7,18-19H,4-6,8H2,1H3/b3-2+
|
|||
| InChIKey |
MXPOCMVWFLDDLZ-NSCUHMNNSA-N
|
|||
| CAS Number |
CAS 114560-48-4
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Human Deoxyribonucleic acid (hDNA) | Target Info | Modulator | [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800000838) | |||
| REF 2 | ClinicalTrials.gov (NCT00598806) Phase 3 Trial of Single-Dose Intravesical EOquin as a Surgical Adjuvant for Noninvasive Bladder Cancer. U.S. National Institutes of Health. | |||
| REF 3 | EO9 (Apaziquone): from the clinic to the laboratory and back again | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

