Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0G2CG
|
|||
| Drug Name |
AL3818
|
|||
| Synonyms |
Anlotinib; 1058156-90-3; UNII-GKF8S4C432; GKF8S4C432; AL-3818; SCHEMBL2063386; GTPL9601; KSMZEXLVHXZPEF-UHFFFAOYSA-N; MolPort-044-567-604; ZINC117924202; AKOS030526233; DB11885; CS-5396; AL 3818; HY-19716; 1-[[4-[(4-fluoro-2-methyl-1H-indol-5-yl)oxy]-6-methoxyquinolin-7-yl]oxymethyl]cyclopropan-1-amine; 1-((4-(4-fluoro-2-methyl-1h-indol-5-yloxy)-6-methoxyquinolin-7-yloxy)methyl)cyclopropan-amine; Cyclopropanamine, 1-(((4-((4-fluoro-2-methyl-1H-indol-5-yl)oxy)-6-methoxy-7-quinolinyl)oxy)methyl)-
Click to Show/Hide
|
|||
| Indication | Alveolar soft part sarcoma [ICD-11: 2A60-2C35] | Phase 3 | [1] | |
| Leiomyosarcoma [ICD-11: 2B58] | Phase 3 | [1] | ||
| Synovial sarcoma [ICD-11: 2B5A; ICD-9: 171] | Phase 3 | [1] | ||
| Cervical cancer [ICD-11: 2C77.0; ICD-9: 180] | Phase 1/2 | [1] | ||
| Endometrial cancer [ICD-11: 2C76; ICD-9: 182] | Phase 1/2 | [1] | ||
| Fallopian tube cancer [ICD-11: 2C74; ICD-10: C57.0] | Phase 1/2 | [1] | ||
| Ovarian cancer [ICD-11: 2C73; ICD-10: C56; ICD-9: 183] | Phase 1/2 | [1], [2] | ||
| Peritoneal cavity cancer [ICD-11: 2C51.Z] | Phase 1/2 | [1] | ||
| Company |
Advenchen Laboratories Moorpark, CA
|
|||
| Structure |
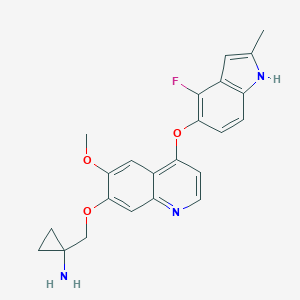 |
Download2D MOL |
||
| Formula |
C23H22FN3O3
|
|||
| Canonical SMILES |
CC1=CC2=C(N1)C=CC(=C2F)OC3=C4C=C(C(=CC4=NC=C3)OCC5(CC5)N)OC
|
|||
| InChI |
1S/C23H22FN3O3/c1-13-9-15-16(27-13)3-4-19(22(15)24)30-18-5-8-26-17-11-21(20(28-2)10-14(17)18)29-12-23(25)6-7-23/h3-5,8-11,27H,6-7,12,25H2,1-2H3
|
|||
| InChIKey |
KSMZEXLVHXZPEF-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1058156-90-3
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

