Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0G3YC
|
|||
| Former ID |
DIB002605
|
|||
| Drug Name |
Netupitant
|
|||
| Synonyms |
R-1124; NK1 antagonist (emesis), Roche; Ro-67-3189
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Chemotherapy-induced nausea [ICD-11: MD90; ICD-10: R11] | Phase 3 | [1], [2] | |
| Company |
Helsinn Therapeutics
|
|||
| Structure |
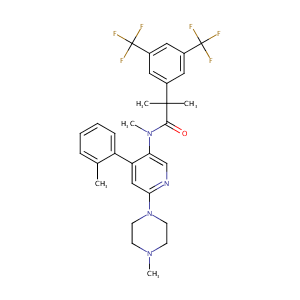 |
Download2D MOL |
||
| Formula |
C30H32F6N4O
|
|||
| Canonical SMILES |
CC1=CC=CC=C1C2=CC(=NC=C2N(C)C(=O)C(C)(C)C3=CC(=CC(=C3)C(F)(F)F)C(F)(F)F)N4CCN(CC4)C
|
|||
| InChI |
1S/C30H32F6N4O/c1-19-8-6-7-9-23(19)24-17-26(40-12-10-38(4)11-13-40)37-18-25(24)39(5)27(41)28(2,3)20-14-21(29(31,32)33)16-22(15-20)30(34,35)36/h6-9,14-18H,10-13H2,1-5H3
|
|||
| InChIKey |
WAXQNWCZJDTGBU-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 290297-26-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
11972336, 15109574, 43042117, 47206876, 50788120, 57370279, 103482309, 104063884, 114526492, 125683129, 126671554, 135246549, 137248756, 142494945, 162830915, 178102368, 179323031, 198988386, 210275497, 210281145, 223666980, 223771059, 226767714, 246332907, 249807117, 250163183, 251971119, 252215904, 252480189
|
|||
| ChEBI ID |
CHEBI:85155
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Substance-P receptor (TACR1) | Target Info | Antagonist | [3] |
| KEGG Pathway | Calcium signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Measles | ||||
| Panther Pathway | CCKR signaling map ST | |||
| Reactome | G alpha (q) signalling events | |||
| WikiPathways | SIDS Susceptibility Pathways | |||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| Spinal Cord Injury | ||||
| Peptide GPCRs | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5742). | |||
| REF 2 | ClinicalTrials.gov (NCT01339260) An Efficacy and Safety Study of Oral Netupitant and Palonosetron for the Prevention of Nausea and Vomiting. U.S. National Institutes of Health. | |||
| REF 3 | Netupitant and palonosetron trigger NK1 receptor internalization in NG108-15 cells. Exp Brain Res. 2014 Aug;232(8):2637-44. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

