Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0H1EA
|
|||
| Former ID |
DNCL002271
|
|||
| Drug Name |
TH-302
|
|||
| Synonyms |
TH-302; evofosfamide; 918633-87-1; TH 302; TH302; UNII-8A9RZ3HN8W; Evofosfamide(TH 302); n,n'-bis(2-bromoethyl)phosphorodiamidic acid (1-methyl-2-nitro-1h-imidazol-5-yl)methyl ester; 8A9RZ3HN8W; compound 3b; TH-302; Evofosfamide;HAP-302; Phosphorodiamidic acid, N,N'-bis(2-bromoethyl)-, (1-methyl-2-nitro-1H-imidazol-5-yl)methyl ester; 2-bromo-N-[(2-bromoethylamino)-[(3-methyl-2-nitroimidazol-4-yl)methoxy]phosphoryl]ethanamine; Evofosfamide [USAN:INN]; Evofosfamide(TH-302); C9H16Br2N5O4P; CHEMBL260046; SCHEMBL2357174
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Soft tissue sarcoma [ICD-11: 2B57; ICD-9: 171] | Phase 2 | [1], [2] | |
| Melanoma [ICD-11: 2C30; ICD-9: 172] | Phase 1 | [3] | ||
| Pancreatic cancer [ICD-11: 2C10] | Phase 1 | [3] | ||
| Prostate cancer [ICD-11: 2C82.0; ICD-10: C61; ICD-9: 185] | Phase 1 | [3] | ||
| Squamous cell carcinoma [ICD-11: 2B60-2D01] | Phase 1 | [3] | ||
| Company |
Threshold Pharmaceuticals
|
|||
| Structure |
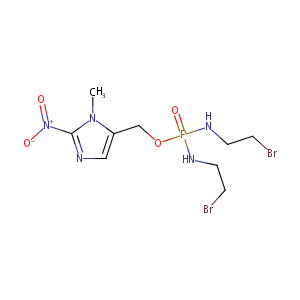 |
Download2D MOL |
||
| Formula |
C9H16Br2N5O4P
|
|||
| Canonical SMILES |
CN1C(=CN=C1[N+](=O)[O-])COP(=O)(NCCBr)NCCBr
|
|||
| InChI |
1S/C9H16Br2N5O4P/c1-15-8(6-12-9(15)16(17)18)7-20-21(19,13-4-2-10)14-5-3-11/h6H,2-5,7H2,1H3,(H2,13,14,19)
|
|||
| InChIKey |
UGJWRPJDTDGERK-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 918633-87-1
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Human Deoxyribonucleic acid (hDNA) | Target Info | Modulator | [4] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8695). | |||
| REF 2 | ClinicalTrials.gov (NCT01403610) Safety and Efficacy Study of TH-302 CNS Penetration in Recurrent High Grade Astrocytoma Following Bevacizumab. U.S. National Institutes of Health. | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | Company report (Threshold Pharmaceuticals) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

