Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0H1YQ
|
|||
| Former ID |
DCL000664
|
|||
| Drug Name |
Xatral
|
|||
| Synonyms |
Alfetim; Alfoten; Urion; Uroxatral; Alfuzosin HCl; Alfuzosin Hydrochloride; Alfuzosin hydrochloride [USAN]; Xatral OD; Xatral Retard; Xatral SR; Xatral XL; SL 77 499-10; SL 77499-10; SL-7749910; Uroxatral, Alfuzosin hydrochloride; Alfuzosin hydrochloride (JAN/USAN); SL-77499-10; SL-77.0499-10; N-[3-[4-amino-6,7-dimethoxy-2-quinazolinyl)methylamino]propyl]tetrahydro-2-furancarboxamide hydrochloride; N-[3-[(4-amino-6,7-dimethoxyquinazolin-2-yl)-methylamino]propyl]oxolane-2-carboxamide hydrochloride; N-{3-[(4-amino-6,7-dimethoxychinazolin-2-yl)(methyl)amino]propyl}tetrahydrofuran-2-carboxamidhydrochlorid; (+-)-N-(3-((4-Amino-6,7-dimethoxy-2-quinazolinyl)methylamino)propyl)tetrahydro-2-furamide monohydrochloride
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Benign prostatic hyperplasia [ICD-11: GA90; ICD-10: N40; ICD-9: 600] | Approved | [1] | |
| Company |
Sanofi-Aventis
|
|||
| Structure |
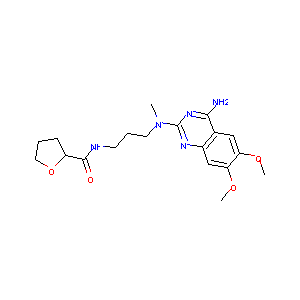 |
Download2D MOL |
||
| Formula |
C19H28ClN5O4
|
|||
| Canonical SMILES |
CN(CCCNC(=O)C1CCCO1)C2=NC3=CC(=C(C=C3C(=N2)N)OC)OC.Cl
|
|||
| InChI |
1S/C19H27N5O4.ClH/c1-24(8-5-7-21-18(25)14-6-4-9-28-14)19-22-13-11-16(27-3)15(26-2)10-12(13)17(20)23-19;/h10-11,14H,4-9H2,1-3H3,(H,21,25)(H2,20,22,23);1H
|
|||
| InChIKey |
YTNKWDJILNVLGX-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 81403-68-1
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
7848755, 8194817, 11528652, 12012888, 14758527, 43128042, 57318350, 78268148, 92125289, 92719118, 99004050, 99437262, 103679622, 103838871, 103914210, 104351250, 117539890, 124757231, 125164035, 125334025, 126621070, 126670423, 134223983, 134339185, 135029857, 135692140, 135698308, 136368114, 144115579, 144204252, 152106189, 162037591, 162179024, 163132546, 163564178, 164814685, 170465022, 175611078, 196110834, 198992200, 203355613, 210279324, 210281647, 223401659, 224081319, 226540787, 251912085, 251915158, 252157178, 252359239
|
|||
| ChEBI ID |
CHEBI:32286
|
|||
| ADReCS Drug ID | BADD_D00070 | |||
| SuperDrug ATC ID |
G04CA01
|
|||
| SuperDrug CAS ID |
cas=081403807
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Adrenergic receptor alpha-1A (ADRA1A) | Target Info | Antagonist | [2] |
| KEGG Pathway | Calcium signaling pathway | |||
| cGMP-PKG signaling pathway | ||||
| Neuroactive ligand-receptor interaction | ||||
| AMPK signaling pathway | ||||
| Adrenergic signaling in cardiomyocytes | ||||
| Vascular smooth muscle contraction | ||||
| Salivary secretion | ||||
| Panther Pathway | Alpha adrenergic receptor signaling pathway | |||
| Reactome | Adrenoceptors | |||
| G alpha (q) signalling events | ||||
| G alpha (12/13) signalling events | ||||
| WikiPathways | Monoamine GPCRs | |||
| Calcium Regulation in the Cardiac Cell | ||||
| GPCRs, Class A Rhodopsin-like | ||||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| Endothelin Pathways | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| AMPK Signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | Pharma & Vaccines. Product Development Pipeline. April 29 2009. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

