Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0H9DA
|
|||
| Former ID |
DIB003378
|
|||
| Drug Name |
AZD6738
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Small-cell lung cancer [ICD-11: 2C25.Y] | Phase 2 | [1] | |
| Chronic lymphocytic leukaemia [ICD-11: 2A82.0; ICD-10: C83.0, C91.1] | Phase 1 | [2] | ||
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C00-D48; ICD-9: 140-199, 210-229] | Phase 1 | [3] | ||
| Company |
Astrazeneca; eagle pharmaceuticals
|
|||
| Structure |
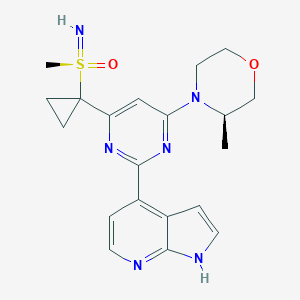 |
Download2D MOL |
||
| Formula |
C20H24N6O2S
|
|||
| Canonical SMILES |
CC1COCCN1C2=NC(=NC(=C2)C3(CC3)S(=N)(=O)C)C4=C5C=CNC5=NC=C4
|
|||
| InChI |
1S/C20H24N6O2S/c1-13-12-28-10-9-26(13)17-11-16(20(5-6-20)29(2,21)27)24-19(25-17)15-4-8-23-18-14(15)3-7-22-18/h3-4,7-8,11,13,21H,5-6,9-10,12H2,1-2H3,(H,22,23)/t13-,29-/m1/s1
|
|||
| InChIKey |
OHUHVTCQTUDPIJ-JYCIKRDWSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Serine/threonine-protein kinase ATR (FRP1) | Target Info | Inhibitor | [4], [5], [6] |
| KEGG Pathway | Fanconi anemia pathway | |||
| Cell cycle | ||||
| p53 signaling pathway | ||||
| HTLV-I infection | ||||
| Panther Pathway | p53 pathway | |||
| p53 pathway feedback loops 2 | ||||
| Pathway Interaction Database | Fanconi anemia pathway | |||
| ATR signaling pathway | ||||
| Signaling events mediated by TCPTP | ||||
| Circadian rhythm pathway | ||||
| BARD1 signaling events | ||||
| p53 pathway | ||||
| Reactome | Meiotic synapsis | |||
| Activation of ATR in response to replication stress | ||||
| Regulation of HSF1-mediated heat shock response | ||||
| HDR through Single Strand Annealing (SSA) | ||||
| Processing of DNA double-strand break ends | ||||
| Presynaptic phase of homologous DNA pairing and strand exchange | ||||
| G2/M DNA damage checkpoint | ||||
| WikiPathways | DNA Damage Response | |||
| Meiotic Synapsis | ||||
| Prostate Cancer | ||||
| Integrated Breast Cancer Pathway | ||||
| Integrated Cancer pathway | ||||
| Cell Cycle | ||||
| Cell Cycle Checkpoints | ||||
| miRNA Regulation of DNA Damage Response | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03428607) Study of AZD6738 and Olaparib Combination Therapy in Relapsed Small Cell Lung Cancer Patients [SUKSES-N2]. U.S. National Institutes of Health. | |||
| REF 2 | ClinicalTrials.gov (NCT01955668) AZD6738 First Time in Patient Multiple Ascending Dose Study. U.S. National Institutes of Health. | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | National Cancer Institute Drug Dictionary (drug id 754022). | |||
| REF 5 | ATR inhibitors VE-821 and VX-970 sensitize cancer cells to topoisomerase i inhibitors by disabling DNA replication initiation and fork elongation responses. Cancer Res. 2014 Dec 1;74(23):6968-79. | |||
| REF 6 | Potentiation of tumor responses to DNA damaging therapy by the selective ATR inhibitor VX-970. Oncotarget. 2014 Jul 30;5(14):5674-85. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

