Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0HE0Y
|
|||
| Drug Name |
Amifampridine
|
|||
| Synonyms |
3,4-DIAMINOPYRIDINE; pyridine-3,4-diamine; 3,4-Pyridinediamine; Firdapse; Diamino-3,4 pyridine; 4,5-Diaminopyridine; 3,4-DAP; SC10; amifampridin; Zenas; UNII-RU4S6E2G0J; EINECS 200-220-9; NSC 521760; BRN 0110232; RU4S6E2G0J; OYTKINVCDFNREN-UHFFFAOYSA-N; WT559; NCGC00167560-01; SMR000752913
Click to Show/Hide
|
|||
| Indication | LambertEaton myasthenic syndrome [ICD-11: 8C62; ICD-10: G73.1] | Approved | [1] | |
| Company |
Catalyst Pharmaceuticals
|
|||
| Structure |
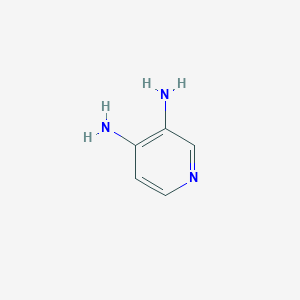 |
Download2D MOL |
||
| Formula |
C5H7N3
|
|||
| Canonical SMILES |
C1=CN=CC(=C1N)N
|
|||
| InChI |
1S/C5H7N3/c6-4-1-2-8-3-5(4)7/h1-3H,7H2,(H2,6,8)
|
|||
| InChIKey |
OYTKINVCDFNREN-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 54-96-6
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:135948
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Potassium channel unspecific (KC) | Target Info | Blocker | [1] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | 2018 FDA drug approvals.Nat Rev Drug Discov. 2019 Feb;18(2):85-89. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

