Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0I1FQ
|
|||
| Former ID |
DAP001510
|
|||
| Drug Name |
Raltegravir
|
|||
| Synonyms |
RGV; MK 0518; Isentress(TM); K-0518; MK-0518; Raltegravir (INN); N-(2-(4-(4-fluorobenzylcarbamoyl); N-((4-Fluorophenyl)methyl)-1,6-dihydro-5-hydroxy-1-methyl-2-(1-methyl-1-(((5-methyl-1,3,4-oxadiazol-2-yl)carbonyl)amino)ethyl)-6-oxo-4-pyrimidinecarboxamide; RAL
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Human immunodeficiency virus infection [ICD-11: 1C62; ICD-9: 279.3] | Approved | [1] | |
| Company |
Merck
|
|||
| Structure |
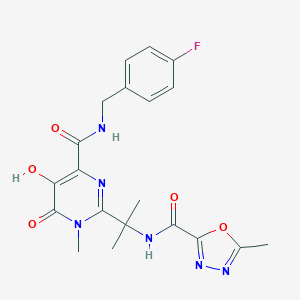 |
Download2D MOL |
||
| Formula |
C20H21FN6O5
|
|||
| Canonical SMILES |
CC1=NN=C(O1)C(=O)NC(C)(C)C2=NC(=C(C(=O)N2C)O)C(=O)NCC3=CC=C(C=C3)F
|
|||
| InChI |
1S/C20H21FN6O5/c1-10-25-26-17(32-10)16(30)24-20(2,3)19-23-13(14(28)18(31)27(19)4)15(29)22-9-11-5-7-12(21)8-6-11/h5-8,28H,9H2,1-4H3,(H,22,29)(H,24,30)
|
|||
| InChIKey |
CZFFBEXEKNGXKS-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 518048-05-0
|
|||
| PubChem Compound ID | ||||
| ADReCS Drug ID | BADD_D01905 ; BADD_D01906 | |||
| SuperDrug ATC ID |
J05AX08
|
|||
| Drug Resistance Mutation (DRM) | Top | |||
|---|---|---|---|---|
| DRM | DRM Info | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Human immunodeficiency virus Integrase (HIV IN) | Target Info | Modulator | [1] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | 2007 FDA drug approvals: a year of flux. Nat Rev Drug Discov. 2008 Feb;7(2):107-9. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

