Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0I2SD
|
|||
| Former ID |
DAP001211
|
|||
| Drug Name |
Medroxyprogesterone
|
|||
| Synonyms |
Asconale; Colirest; Hematrol; Hydroxymethylprogesterone; Lunelle; Medrossiprogesterone; Medroxiprogesterona; Medroxiprogesteronum; Medroxyprogesteron; Medroxyprogesteronum; Methylhydroxyprogesterone; Adgyn Medro; Farlutal inyectable; Medroprogesterone Acetate; Medrossiprogesterone [DCIT]; Medroxyprogesteron acetate; Medroxyprogesterone Strakan Brand; Sodelut G; Strakan Brand of Medroxyprogesterone; MPA Gyn 5; U 8840; CBP-1011; Farlutal inyectable (TN); G-Farlutal; Medroxiprogesterona [INN-Spanish]; Medroxyprogesterone (INN); Medroxyprogesterone [INN:BAN]; Medroxyprogesteronum [INN-Latin]; Novo-Medrone; Pregn-4-ene-3,20-dione, 17-hydroxy-6-methyl-, (6alpha)-(9CI); Pregn-4-ene-3,20-dione, 17-hydroxy-6-methyl-, (6-alpha)-(9CI); (6S,8R,9S,10R,13S,14S,17R)-17-acetyl-17-hydroxy-6,10,13-trimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-3-one; (6alpha)-17-hydroxy-6-methylpregn-4-ene-3,20-dione; 17 alpha Hydroxy 6 alpha Methylprogesterone; 17 alpha-Hydroxy-6 alpha-Methylprogesterone; 17-Hydroxy-6.alpha.-methylprogesterone; 17-Hydroxy-6alpha-methyl-pregn-4-ene-3,20-dione; 17-Hydroxy-6alpha-methylprogesterone; 17.alpha.-Hydroxy-6.alpha.-methylprogesterone; 17alpha-Hydroxy-6alpha-methyl-4-pregnene-3,20-dione; 17alpha-Hydroxy-6alpha-methylprogesterone; 6-Dihydromegestrol; 6-alpha-Methyl-17-alpha-hydroxyprogesterone; 6.alpha.-Methyl-17.alpha.-hydroxyprogesterone; 6alpha-Methyl-17alpha-hydroxyprogesterone; 6alpha-Methyl-4-pregnen-17alpha-ol-3,20-dione; 6alpha-Methyl-5-pregnen-17alpha-ol-3,20-dione
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C76-C80; ICD-9: 140-229] | Approved | [1] | |
| Therapeutic Class |
Contraceptive Agents
|
|||
| Company |
Pharmacia And Upjohn Co
|
|||
| Structure |
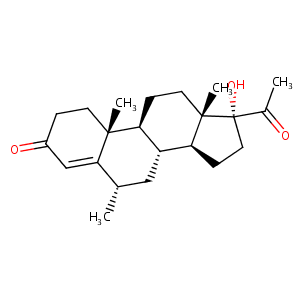 |
Download2D MOL |
||
| Formula |
C22H32O3
|
|||
| Canonical SMILES |
CC1CC2C(CCC3(C2CCC3(C(=O)C)O)C)C4(C1=CC(=O)CC4)C
|
|||
| InChI |
1S/C22H32O3/c1-13-11-16-17(20(3)8-5-15(24)12-19(13)20)6-9-21(4)18(16)7-10-22(21,25)14(2)23/h12-13,16-18,25H,5-11H2,1-4H3/t13-,16+,17-,18-,20+,21-,22-/m0/s1
|
|||
| InChIKey |
FRQMUZJSZHZSGN-HBNHAYAOSA-N
|
|||
| CAS Number |
CAS 520-85-4
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9330, 87898, 855830, 7979879, 8157679, 14851694, 14875913, 24870138, 24897065, 29215011, 29229105, 46387018, 50019696, 50743697, 53790835, 56352890, 56422093, 57326352, 57650792, 71970578, 85300839, 92308558, 92713474, 95613571, 96024856, 103469393, 104323836, 124583837, 124659090, 124799312, 126524241, 126629791, 126656028, 127339982, 127339983, 127889793, 134338451, 134977029, 135650577, 137004153, 137188138, 141792672, 144089042, 144206952, 152101100, 160963948, 163135668, 164761742, 170466063, 172080484
|
|||
| ChEBI ID |
CHEBI:6715
|
|||
| ADReCS Drug ID | BADD_D01365 | |||
| SuperDrug ATC ID |
G03AC06; G03DA02; L02AB02
|
|||
| SuperDrug CAS ID |
cas=000520854
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Progesterone receptor (PGR) | Target Info | Agonist | [2] |
| KEGG Pathway | Oocyte meiosis | |||
| Progesterone-mediated oocyte maturation | ||||
| Pathway Interaction Database | Cellular roles of Anthrax toxin | |||
| Reactome | Nuclear signaling by ERBB4 | |||
| Nuclear Receptor transcription pathway | ||||
| WikiPathways | Ovarian Infertility Genes | |||
| Signaling by ERBB4 | ||||
| Nuclear Receptors | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | Dienogest is a selective progesterone receptor agonist in transactivation analysis with potent oral endometrial activity due to its efficient pharm... Steroids. 2008 Feb;73(2):222-31. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

