Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0I5DP
|
|||
| Drug Name |
AMG 176
|
|||
| Synonyms |
JQNINBDKGLWYMU-GEAQBIRJSA-N; AMG-176; 1883727-34-1; AMG176; SCHEMBL17550216; EX-A2666; HY-101565; CS-0021721; Spiro[5,7-etheno-1H,11H-cyclobut[i][1,4]oxazepino[3,4-f][1,2,7]thiadiazacyclohexadecine-2(3H),1'(2'H)-naphthalen]-8(9H)-one, 6'-chloro-3',4',12,13,16,16a,17,18,18a,19-decahydro-16-methoxy-11,12-dimethyl-,10,10-dioxide, (1'S,11R,12S,14E,16S,16aR,18aR)-
Click to Show/Hide
|
|||
| Indication | Acute myeloid leukaemia [ICD-11: 2A60; ICD-9: 205] | Phase 1 | [1] | |
| Multiple myeloma [ICD-11: 2A83; ICD-10: C90.0] | Phase 1 | [2] | ||
| Company |
AmgenThousand Oaks, CA
|
|||
| Structure |
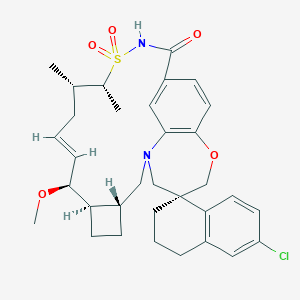 |
Download2D MOL |
||
| Formula |
C33H41ClN2O5S
|
|||
| Canonical SMILES |
CC1CC=CC(C2CCC2CN3CC4(CCCC5=C4C=CC(=C5)Cl)COC6=C3C=C(C=C6)C(=O)NS(=O)(=O)C1C)OC
|
|||
| InChI |
1S/C33H41ClN2O5S/c1-21-6-4-8-30(40-3)27-12-9-25(27)18-36-19-33(15-5-7-23-16-26(34)11-13-28(23)33)20-41-31-14-10-24(17-29(31)36)32(37)35-42(38,39)22(21)2/h4,8,10-11,13-14,16-17,21-22,25,27,30H,5-7,9,12,15,18-20H2,1-3H3,(H,35,37)/b8-4+/t21-,22+,25-,27+,30-,33-/m0/s1
|
|||
| InChIKey |
JQNINBDKGLWYMU-GEAQBIRJSA-N
|
|||
| CAS Number |
CAS 1883727-34-1
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Induced myeloid leukemia cell differentiation protein Mcl-1 (MCL1) | Target Info | Inhibitor | [2] |
| KEGG Pathway | PI3K-Akt signaling pathway | |||
| MicroRNAs in cancer | ||||
| NetPath Pathway | TCR Signaling Pathway | |||
| Panther Pathway | Apoptosis signaling pathway | |||
| CCKR signaling map ST | ||||
| Pathway Interaction Database | E2F transcription factor network | |||
| Direct p53 effectors | ||||
| IL6-mediated signaling events | ||||
| HIF-1-alpha transcription factor network | ||||
| WikiPathways | Apoptosis | |||
| miR-targeted genes in muscle cell - TarBase | ||||
| miR-targeted genes in lymphocytes - TarBase | ||||
| miR-targeted genes in leukocytes - TarBase | ||||
| Apoptosis Modulation and Signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03797261) A Study of Venetoclax and AMG 176 in Patients With Relapsed/Refractory Hematologic Malignancies. U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

