Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0I6ZO
|
|||
| Former ID |
DIB013334
|
|||
| Drug Name |
AZD-5213
|
|||
| Indication | Alzheimer disease [ICD-11: 8A20; ICD-10: G30, G30.9; ICD-9: 331] | Phase 2 | [1] | |
| Company |
AstraZeneca plc
|
|||
| Structure |
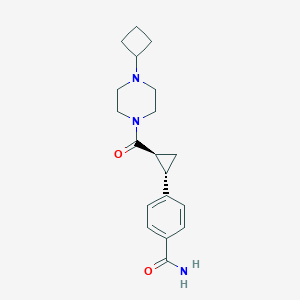 |
Download2D MOL |
||
| Formula |
C19H25N3O2
|
|||
| Canonical SMILES |
C1CC(C1)N2CCN(CC2)C(=O)C3CC3C4=CC=C(C=C4)C(=O)N
|
|||
| InChI |
1S/C19H25N3O2/c20-18(23)14-6-4-13(5-7-14)16-12-17(16)19(24)22-10-8-21(9-11-22)15-2-1-3-15/h4-7,15-17H,1-3,8-12H2,(H2,20,23)/t16-,17+/m1/s1
|
|||
| InChIKey |
VCQZCDSEWSFTPO-SJORKVTESA-N
|
|||
| CAS Number |
CAS 1119807-02-1
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Histamine H3 receptor (H3R) | Target Info | Antagonist | [2] |
| KEGG Pathway | Neuroactive ligand-receptor interaction | |||
| Reactome | Histamine receptors | |||
| G alpha (i) signalling events | ||||
| WikiPathways | Monoamine Transport | |||
| GPCRs, Class A Rhodopsin-like | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT01548287) A Study of the Safety and Tolerability of AZD5213 Effect on Sleep for Patients With Alzheimer's/Cognitive Impairment. U.S. National Institutes of Health. | |||
| REF 2 | SAR110894, a potent histamine H receptor antagonist, displays procognitive effects in rodents. Pharmacol Biochem Behav. 2012 Aug;102(2):203-14. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

