Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0I9AT
|
|||
| Former ID |
DIB021092
|
|||
| Drug Name |
tryptanthrin
|
|||
| Synonyms |
GNF-PF-2691; couroupitine a
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
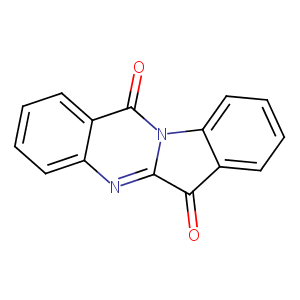 |
Download2D MOL
|
||
| Formula |
C15H8N2O2
|
|||
| Canonical SMILES |
C1=CC=C2C(=C1)C(=O)N3C4=CC=CC=C4C(=O)C3=N2
|
|||
| InChI |
1S/C15H8N2O2/c18-13-10-6-2-4-8-12(10)17-14(13)16-11-7-3-1-5-9(11)15(17)19/h1-8H
|
|||
| InChIKey |
VQQVWGVXDIPORV-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 13220-57-0
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
12925, 463068, 606847, 872097, 4453145, 8195762, 11407505, 15196648, 24823948, 26754607, 43129468, 47737193, 50110078, 50354465, 53837731, 57319147, 57727906, 81092989, 85090210, 85860316, 90460831, 103274177, 103850092, 104098377, 104355519, 105218556, 117357400, 124548921, 124813199, 129513211, 131334994, 135033181, 137171547, 142307813, 160836366, 162091701, 163305319, 163399256, 164235665, 170179247, 172904085, 175607820, 184573636, 186011300, 204370956, 223251841, 223441148, 223454999, 223485542, 223519501
|
|||
| ChEBI ID |
CHEBI:9768
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Indoleamine 2,3-dioxygenase 1 (IDO1) | Target Info | Inhibitor | [1] |
| BioCyc | Superpathway of tryptophan utilization | |||
| Tryptophan degradation | ||||
| L-kynurenine degradation | ||||
| Tryptophan degradation to 2-amino-3-carboxymuconate semialdehyde | ||||
| NAD de novo biosynthesis | ||||
| KEGG Pathway | Tryptophan metabolism | |||
| Metabolic pathways | ||||
| African trypanosomiasis | ||||
| NetPath Pathway | TSLP Signaling Pathway | |||
| IL5 Signaling Pathway | ||||
| TGF_beta_Receptor Signaling Pathway | ||||
| Pathwhiz Pathway | Tryptophan Metabolism | |||
| Reactome | Tryptophan catabolism | |||
| WikiPathways | Tryptophan metabolism | |||
| Metabolism of amino acids and derivatives | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Discovery of tryptanthrin derivatives as potent inhibitors of indoleamine 2,3-dioxygenase with therapeutic activity in Lewis lung cancer (LLC) tumor-bearing mice. J Med Chem. 2013 Nov 14;56(21):8321-31. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

