Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0J5KF
|
|||
| Former ID |
DAP000295
|
|||
| Drug Name |
Granisetron
|
|||
| Synonyms |
BRL-43694; Kytril (TN); Granisetron (USAN/INN); 1-methyl-N-[(1S,5R)-9-methyl-9-azabicyclo[3.3.1]nonan-3-yl]indazole-3-carboxamide; 1-methyl-N-[(3-endo)-9-methyl-9-azabicyclo[3.3.1]non-3-yl]-1H-indazole-3-carboxamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Nausea and vomiting [ICD-11: MD90; ICD-10: R11] | Approved | [1] | |
| Therapeutic Class |
Antiemetics
|
|||
| Company |
Roche Pharmaceuticals
|
|||
| Structure |
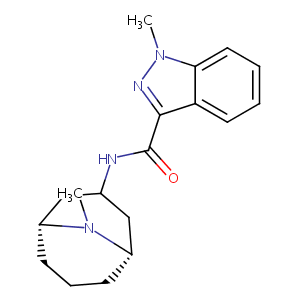 |
Download2D MOL |
||
| Formula |
C18H24N4O
|
|||
| Canonical SMILES |
CN1C2CCCC1CC(C2)NC(=O)C3=NN(C4=CC=CC=C43)C
|
|||
| InChI |
1S/C18H24N4O/c1-21-13-6-5-7-14(21)11-12(10-13)19-18(23)17-15-8-3-4-9-16(15)22(2)20-17/h3-4,8-9,12-14H,5-7,10-11H2,1-2H3,(H,19,23)/t12?,13-,14+
|
|||
| InChIKey |
MFWNKCLOYSRHCJ-AGUYFDCRSA-N
|
|||
| CAS Number |
CAS 109889-09-0
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9235, 7979461, 11039351, 14752282, 14776668, 37101819, 39317881, 47206270, 48416070, 50304713, 56313746, 57359119, 92308990, 93166430, 103633664, 104133250, 113863200, 126616777, 126651441, 131294324, 134337607, 135017388, 135650322, 135795502, 135809187, 136967344, 137002702, 137187129, 137248570, 139842644, 141065090, 141065156, 142399895, 160650426, 164810730, 170507519, 175268823, 175612170, 176484239, 179149548, 179296054, 184021080, 184812331, 194147904, 194941564, 198991523, 206246341, 223439717, 223654566, 225144985
|
|||
| ChEBI ID |
CHEBI:5537
|
|||
| ADReCS Drug ID | BADD_D01040 | |||
| SuperDrug ATC ID |
A04AA02
|
|||
| SuperDrug CAS ID |
cas=109889090
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | 5-HT 3 receptor (5HT3R) | Target Info | Antagonist | [2], [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | New approaches to chemotherapy-induced nausea and vomiting: from neuropharmacology to clinical investigations. Cancer J. 2006 Sep-Oct;12(5):341-7. | |||
| REF 3 | Reduction of rectal sensitivity and post-prandial motility by granisetron, a 5 HT3-receptor antagonist, in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 1993 Apr;7(2):175-80. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

