Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0J8KW
|
|||
| Former ID |
DNC001372
|
|||
| Drug Name |
SR144528
|
|||
| Synonyms |
SR 144528; SR144528; 192703-06-3; SR-144528; CHEMBL381791; 5-(4-Chloro-3-methylphenyl)-1-((4-methylphenyl)methyl)-N-(1,3,3-trimethylbicyclo(2.2.1)hept-2-yl)-1H-pyrazole-3-carboxamide (1S-endo)-; SR144,528; (1S-endo)-5-(4-Chloro-3-methylphenyl)-1-((4-methylphenyl)methyl)-N-(1,3,3-trimethylbicyclo(2.2.1)hept-2-yl)-1H-pyrazole-3-carboxamide; 5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-N-((1S,2S,4R)-1,3,3-trimethylbicyclo[2.2.1]heptan-2-yl)-1H-pyrazole-3-carboxamide; 1H-Pyrazole-3-carboxamide, 5-(4-chloro-3-methylphe
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
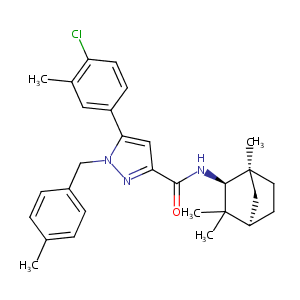 |
Download2D MOL |
||
| Formula |
C29H34ClN3O
|
|||
| Canonical SMILES |
CC1=CC=C(C=C1)CN2C(=CC(=N2)C(=O)NC3C(C4CCC3(C4)C)(C)C)C5=CC(=C(C=C5)Cl)C
|
|||
| InChI |
1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34)/t22-,27-,29+/m1/s1
|
|||
| InChIKey |
SUGVYNSRNKFXQM-XRHWURSXSA-N
|
|||
| CAS Number |
CAS 192703-06-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ChEBI ID |
CHEBI:146245
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Cannabinoid receptor 2 (CB2) | Target Info | Antagonist | [2] |
| KEGG Pathway | Neuroactive ligand-receptor interaction | |||
| Reactome | Class A/1 (Rhodopsin-like receptors) | |||
| G alpha (i) signalling events | ||||
| WikiPathways | GPCRs, Class A Rhodopsin-like | |||
| Small Ligand GPCRs | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 751). | |||
| REF 2 | Antinociceptive activity of the endogenous fatty acid amide, palmitylethanolamide. Eur J Pharmacol. 2001 May 11;419(2-3):191-8. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

