Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0JN5N
|
|||
| Former ID |
DNCL001703
|
|||
| Drug Name |
Laquinimod
|
|||
| Synonyms |
Laquinimod; 248281-84-7; 5-CHLORO-N-ETHYL-4-HYDROXY-1-METHYL-2-OXO-N-PHENYL-1,2-DIHYDROQUINOLINE-3-CARBOXAMIDE; ABR-215062; ABR 215062; 5-Chloro-4-hydroxy-1-methyl-2-oxo-1,2-dihydro-quinoline-3-carboxylic acid ethyl-phenyl-amide; UNII-908SY76S4G; CIVENTICHEM CV-4057; Laquinimod (ABR-215062); 908SY76S4G; N-Ethyl-N-phenyl-1,2-dihydro-4-hydroxy-5-chloro-1-methyl-2-oxoquinoline-3-carboxamide; 5-chloro-n-ethyl-2-hydroxy-1-methyl-4-oxo-n-phenyl-1,4-dihydroquinoline-3-carboxamide; C19H17ClN2O3
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Lupus [ICD-11: 4A40; ICD-9: 583.81] | Phase 3 | [1], [2] | |
| Multiple sclerosis [ICD-11: 8A40; ICD-9: 340] | Phase 3 | [3], [4] | ||
| Huntington disease [ICD-11: 8A01.10; ICD-10: G10; ICD-9: 294.1, 333.4] | Phase 2 | [4] | ||
| Primary progressive multiple sclerosis [ICD-11: 8A40.1; ICD-9: 340] | Phase 2 | [3] | ||
| Company |
Teva Neuroscience
|
|||
| Structure |
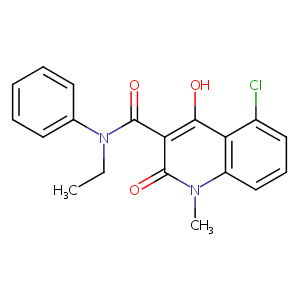 |
Download2D MOL |
||
| Formula |
C19H17ClN2O3
|
|||
| Canonical SMILES |
CCN(C1=CC=CC=C1)C(=O)C2=C(C3=C(C=CC=C3Cl)N(C2=O)C)O
|
|||
| InChI |
1S/C19H17ClN2O3/c1-3-22(12-8-5-4-6-9-12)19(25)16-17(23)15-13(20)10-7-11-14(15)21(2)18(16)24/h4-11,23H,3H2,1-2H3
|
|||
| InChIKey |
GKWPCEFFIHSJOE-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 248281-84-7
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
14876590, 30420153, 39226871, 53789660, 55415688, 57399789, 80696753, 93309883, 103144721, 103258758, 109693522, 113461713, 117465054, 125580534, 126659568, 126671487, 127968532, 135135025, 135684163, 136375556, 136920402, 136946440, 137003754, 137267594, 142657125, 144116129, 152159598, 152254807, 152258415, 152343588, 160817546, 162011445, 162036243, 162192335, 163090687, 163686051, 164761535, 170503204, 172092778, 174007071, 174477470, 174528534, 179150222, 180672675, 185974774, 185974775, 185974776, 198987470, 204386312, 223365974
|
|||
| ChEBI ID |
CHEBI:134738
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7639). | |||
| REF 2 | ClinicalTrials.gov (NCT01047319) A Study to Evaluate the Long-term Safety, Tolerability and Effect of Daily Oral Laquinimod 0.6 mg on Disease Course in Subjects With Relapsing Multiple Sclerosis. U.S. National Institutes of Health. | |||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 4 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 5 | Reduced astrocytic NF- B activation by laquinimod protects from cuprizone-induced demyelination. Acta Neuropathol. 2012 Sep;124(3):411-24. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

