Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0K3OK
|
|||
| Drug Name |
Difelikefalin
|
|||
| Synonyms |
UNII-NA1U919MRO; NA1U919MRO; MR13A9; 1024828-77-0; FE202845; Difelikefalin [INN]; Difelikefalin [USAN:INN]; SEQ ID NO: 2; GTPL9044; CHEMBL3989915; SCHEMBL10316464; BDBM235785; DB11938; FE-202845; US9359399, 2; D-Phe-D-Phe-D-Leu-D-Lys-[gamma-(4-N-piperidinyl)amino carboxylic acid]; 4-Piperidinecarboylic acid, 4-amino-1-(D-phenylalanyl-D-phenylalanyl-D-leucyl-D-lysyl)-; 4-Piperidinecarboxylic acid, N1-(D-phenylalanyl-D-phenylalanyl-D-leucyl-D-lysyl)-4-amino-; 4-Piperidinecarboxylic acid, N1-(D
Click to Show/Hide
|
|||
| Indication | Pruritus [ICD-11: EC90; ICD-10: L29, L29.9; ICD-9: 698] | Approved | [1] | |
| Company |
Cara Therapeutics
|
|||
| Structure |
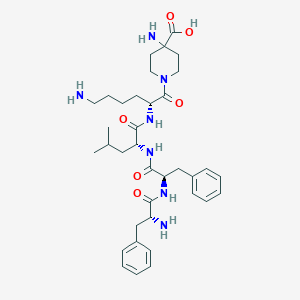 |
Download2D MOL |
||
| Formula |
C36H53N7O6
|
|||
| Canonical SMILES |
CC(C)CC(C(=O)NC(CCCCN)C(=O)N1CCC(CC1)(C(=O)O)N)NC(=O)C(CC2=CC=CC=C2)NC(=O)C(CC3=CC=CC=C3)N
|
|||
| InChI |
1S/C36H53N7O6/c1-24(2)21-29(32(45)40-28(15-9-10-18-37)34(47)43-19-16-36(39,17-20-43)35(48)49)42-33(46)30(23-26-13-7-4-8-14-26)41-31(44)27(38)22-25-11-5-3-6-12-25/h3-8,11-14,24,27-30H,9-10,15-23,37-39H2,1-2H3,(H,40,45)(H,41,44)(H,42,46)(H,48,49)/t27-,28-,29-,30-/m1/s1
|
|||
| InChIKey |
FWMNVWWHGCHHJJ-SKKKGAJSSA-N
|
|||
| CAS Number |
CAS 1024828-77-0
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Opioid receptor kappa (OPRK1) | Target Info | Agonist | [2] |
| KEGG Pathway | Neuroactive ligand-receptor interaction | |||
| Panther Pathway | Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | |||
| Heterotrimeric G-protein signaling pathway-Gq alpha and Go alpha mediated pathway | ||||
| Opioid prodynorphin pathway | ||||
| Reactome | Peptide ligand-binding receptors | |||
| G alpha (i) signalling events | ||||
| WikiPathways | GPCRs, Class A Rhodopsin-like | |||
| Peptide GPCRs | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2021. Application Number: 214916. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

