Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0KC2Z
|
|||
| Former ID |
DNC002939
|
|||
| Drug Name |
Uridine-Diphosphate-N-Acetylglucosamine
|
|||
| Synonyms |
UDP-N-acetylglucosamine; UDP-GlcNAc; UDP-N-acetyl-D-glucosamine; URIDINE-DIPHOSPHATE-N-ACETYLGLUCOSAMINE; UPPAG; UDP-acetylglucosamine; uridine diphosphate N-acetylglucosamine; UDP-N-acetyl-alpha-D-glucosamine; Uridine diphosphate-N-acetylglucosamine; 528-04-1; UDP-acetyl-D-glucosamine; Uridine diphospho-N-acetylglucosamine; Uridine 5'-diphospho-N-acetylglucosamine; [(2R,3R,4R,5S,6R)-3-acetamido-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl] [[(2R,3S,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxy
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
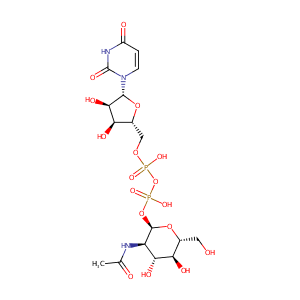 |
Download2D MOL |
||
| Formula |
C17H27N3O17P2
|
|||
| Canonical SMILES |
CC(=O)NC1C(C(C(OC1OP(=O)(O)OP(=O)(O)OCC2C(C(C(O2)N3C=CC(=O)NC3=O)O)O)CO)O)O
|
|||
| InChI |
1S/C17H27N3O17P2/c1-6(22)18-10-13(26)11(24)7(4-21)35-16(10)36-39(31,32)37-38(29,30)33-5-8-12(25)14(27)15(34-8)20-3-2-9(23)19-17(20)28/h2-3,7-8,10-16,21,24-27H,4-5H2,1H3,(H,18,22)(H,29,30)(H,31,32)(H,19,23,28)/t7-,8-,10-,11-,12-,13-,14-,15-,16-/m1/s1
|
|||
| InChIKey |
LFTYTUAZOPRMMI-CFRASDGPSA-N
|
|||
| CAS Number |
CAS 528-04-1
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
3345, 819902, 820180, 820513, 822243, 822377, 822379, 822380, 823804, 823812, 825931, 825934, 7890974, 7890975, 10299750, 11532680, 11532683, 15944818, 24277171, 24277175, 24398025, 24771374, 24778852, 24778858, 26711748, 26716839, 26737131, 26737150, 26737154, 36888309, 46504310, 49658391, 49658394, 57288147, 57404699, 76654218, 92708740, 104634797, 111978669, 125003641, 125003645, 126523139, 134464862, 134464955, 134464959, 135631643, 135642834, 135651588, 137240157, 137253054
|
|||
| ChEBI ID |
CHEBI:16264
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Bacterial UDP-N-acetylglucosamine carboxyvinyltransferase (Bact murA) | Target Info | Inhibitor | [2] |
| UDP-glucose 4-epimerase (GALE) | Target Info | Inhibitor | [2] | |
| BioCyc | D-galactose degradation V (Leloir pathway) | |||
| UDP-N-acetyl-D-galactosamine biosynthesis I | ||||
| UDP-N-acetyl-D-galactosamine biosynthesis II | ||||
| KEGG Pathway | Galactose metabolism | |||
| Amino sugar and nucleotide sugar metabolism | ||||
| Metabolic pathways | ||||
| NetPath Pathway | IL2 Signaling Pathway | |||
| Panther Pathway | Fructose galactose metabolism | |||
| Pathwhiz Pathway | Nucleotide Sugars Metabolism | |||
| Galactose Metabolism | ||||
| WikiPathways | Metabolism of carbohydrates | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1779). | |||
| REF 2 | DrugBank 3.0: a comprehensive resource for 'omics' research on drugs. Nucleic Acids Res. 2011 Jan;39(Database issue):D1035-41. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

