Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0KR2J
|
|||
| Former ID |
DAP000697
|
|||
| Drug Name |
Entecavir
|
|||
| Synonyms |
Baraclude; ETV; Entecavir hydrate; Entecavir monohydrate; BMS-200475; Baraclude (TN); Entecavir (INN); Entecavir (USAN); Entecavir hydrate (JAN); SQ-34676; 2-amino-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]-6H-purin-6-one; 2-amino-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylidenecyclopentyl]-1,9-dihydro-6H-purin-6-one; 2-amino-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylidenecyclopentyl]-1,9-dihydro-6H-purin-6-one-water (1/1); 2-amino-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylidenecyclopentyl]-3H-purin-6-one; 6-H-Purin-6-one-,2-amino-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]; 9-((1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl)guanine monohydrate
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Hepatitis B virus infection [ICD-11: 1E51.0; ICD-10: B18.1] | Approved | [1] | |
| Therapeutic Class |
Antiviral Agents
|
|||
| Company |
Bristol-Myers Squibb
|
|||
| Structure |
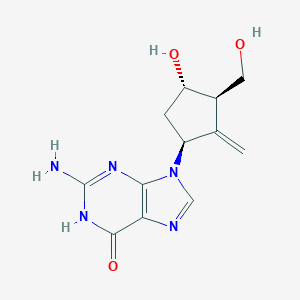 |
Download2D MOL |
||
| Formula |
C12H15N5O3
|
|||
| Canonical SMILES |
C=C1C(CC(C1CO)O)N2C=NC3=C2N=C(NC3=O)N
|
|||
| InChI |
1S/C12H15N5O3/c1-5-6(3-18)8(19)2-7(5)17-4-14-9-10(17)15-12(13)16-11(9)20/h4,6-8,18-19H,1-3H2,(H3,13,15,16,20)/t6-,7-,8-/m0/s1
|
|||
| InChIKey |
QDGZDCVAUDNJFG-FXQIFTODSA-N
|
|||
| CAS Number |
CAS 142217-69-4
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
17398002, 37101953, 49830936, 56391942, 57379683, 91749140, 99319526, 104253461, 118313779, 124757591, 125001900, 125164395, 126651626, 134222146, 136920321, 137129051, 137179356, 152034465, 162011500, 162183301, 163370951, 163908003, 164825059, 164833113, 172087090, 172440023, 174531851, 175265673, 180672743, 186013131, 196106268, 198991663, 210274904, 210280539, 223379249, 223398838, 226415922, 249739265, 251893489, 252110220, 252162333, 252316076
|
|||
| ChEBI ID |
CHEBI:473990
|
|||
| ADReCS Drug ID | BADD_D00774 | |||
| SuperDrug ATC ID |
J05AF10
|
|||
| Drug Resistance Mutation (DRM) | Top | |||
|---|---|---|---|---|
| DRM | DRM Info | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Hepatitis B virus Reverse transcriptase (HBV RT) | Target Info | Modulator | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | |||
| REF 2 | Effect of hepatitis B virus reverse transcriptase variations on entecavir treatment response. J Infect Dis. 2014 Sep 1;210(5):701-7. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

