Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0L0ZF
|
|||
| Former ID |
DNC003894
|
|||
| Drug Name |
VESNARINONE
|
|||
| Synonyms |
vesnarinone; 81840-15-5; Arkin-Z; Piteranometozine; OPC-8212; Arkin; Vesnarinonum [Latin]; Vesnarinona [Spanish]; UNII-5COW40EV8M; OPC 8212; Vesnarinone [USAN:INN:JAN]; CCRIS 1451; DRG-0210; BRN 5644229; 5COW40EV8M; CHEMBL17423; 6-(4-(3,4-Dimethoxybenzoyl)-1-piperazinyl)-3,4-dihydro-2(1H)-; 1-(1,2,3,4-Tetrahydro-2-oxo-6-quinolyl)-4-veratroylpiperazine; 6-[4-(3,4-dimethoxybenzoyl)piperazin-1-yl]-3,4-dihydro-1H-quinolin-2-one; 3,4-Dihydro-6-(4-(3,4-dimethoxybenzoyl)-1-piperazinyl)-2(1H)-quinolinone
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Cardiac failure [ICD-11: BD10-BD13; ICD-10: I50, I50.9] | Approved | [1] | |
| Structure |
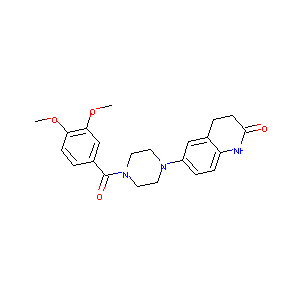 |
Download2D MOL |
||
| Formula |
C22H25N3O4
|
|||
| Canonical SMILES |
COC1=C(C=C(C=C1)C(=O)N2CCN(CC2)C3=CC4=C(C=C3)NC(=O)CC4)OC
|
|||
| InChI |
1S/C22H25N3O4/c1-28-19-7-3-16(14-20(19)29-2)22(27)25-11-9-24(10-12-25)17-5-6-18-15(13-17)4-8-21(26)23-18/h3,5-7,13-14H,4,8-12H2,1-2H3,(H,23,26)
|
|||
| InChIKey |
ZVNYJIZDIRKMBF-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 81840-15-5
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:31237
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Phosphodiesterase 3A (PDE3A) | Target Info | Inhibitor | [2] |
| Voltage-gated potassium channel Kv11.1 (KCNH2) | Target Info | Inhibitor | [3] | |
| KEGG Pathway | Purine metabolism | |||
| cGMP-PKG signaling pathway | ||||
| cAMP signaling pathway | ||||
| Morphine addiction | ||||
| Pathwhiz Pathway | Muscle/Heart Contraction | |||
| Reactome | cGMP effects | |||
| G alpha (s) signalling events | ||||
| Voltage gated Potassium channels | ||||
| WikiPathways | miR-targeted genes in muscle cell - TarBase | |||
| miR-targeted genes in lymphocytes - TarBase | ||||
| SIDS Susceptibility Pathways | ||||
| Hematopoietic Stem Cell Differentiation | ||||
| Potassium Channels | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | Design, synthesis and biological evaluation of 6-(benzyloxy)-4-methylquinolin-2(1H)-one derivatives as PDE3 inhibitors. Bioorg Med Chem. 2010 Jan 15;18(2):855-62. | |||
| REF 3 | Prediction of hERG potassium channel affinity by traditional and hologram qSAR methods. Bioorg Med Chem Lett. 2003 Aug 18;13(16):2773-5. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

