Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0L9ZR
|
|||
| Former ID |
DAP000695
|
|||
| Drug Name |
Praziquantel
|
|||
| Synonyms |
Azinox; Biliricide; Biltricide; Cesol; Cisticid; Cutter; Cysticide; Droncit; Drontsit; Prasiquantel; Praziquantelum; Pyquiton; Traziquantel; Bayer Brand of Praziquantel; Cutter Tape Tabs; Merck Brand of Praziquantel; Embay 8440; P 4668; Bay-8440; Biltricide (TN); EMBAY-8440; NPFAPI-02; Praziquantelum [INN-Latin]; Biltricide, Droncit, Praziquantel; Praziquantel (JAN/USP/INN); Praziquantel [USAN:INN:BAN:JAN]; Praziquantel, (R)-Isomer; Praziquantel, (S)-Isomer; Praziquantel, (+-)-Isomer; (+-)-2-(Cyclohexylcarbonyl)-1,2,3,6,7,11b-hexahydro-4H-pyrazino(2,1a)isoquinolin-4-one; (11bS)-2-(cyclohexylcarbonyl)-1,2,3,6,7,11b-hexahydro-4H-pyrazino[2,1-a]isoquinolin-4-one; 2-(Cyclohexylcarbonyl)-1,2,3,6,7,11b-hexahydro-4H-pyrazino(2,1-a)isoquinolin-4-one; 2-(Cyclohexylcarbonyl)-1,2,3,6,7,11b-hexahydro-4H-pyrazino[2,1-a]-isoquinolin-4-one; 2-(Cyclohexylcarbonyl)-1,2,3,6,7-11b-hexahydro-4H-pyrazino(2,1a)isoquinolin-4-one; 2-(Cyclohexylcarbonyl)-1,2,3,6,7-11b-hexahydro-4H-pyrazinoe(2,1a)isoquinolin-4-one; 2-(cyclohexanecarbonyl)-3,6,7,11b-tetrahydro-1H-pyrazino[2,1-a]isoquinolin-4-one; 2-(cyclohexylcarbonyl)-1,2,3,6,7,11b-hexahydro-4H-pyrazino[2,1-a]isoquinolin-4-one; 2-Cyclohexanecarbonyl-1,2,3,6,7,11b-hexahydro-pyrazino[2,1-a]isoquinolin-4-one; 2-Cyclohexylcarbonyl-1,2,3,6,7,11b-hexahydro-4H-pyrazino(2,1-a) isoquinolin-4-one; 8440, EMBAY
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Flatworm infection [ICD-11: 1F70-1F86] | Approved | [1] | |
| Therapeutic Class |
Anthelmintics
|
|||
| Company |
Bayer Pharmaceuticals Corporation
|
|||
| Structure |
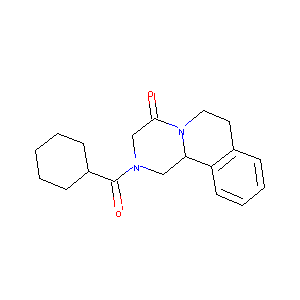 |
Download2D MOL |
||
| Formula |
C19H24N2O2
|
|||
| Canonical SMILES |
C1CCC(CC1)C(=O)N2CC3C4=CC=CC=C4CCN3C(=O)C2
|
|||
| InChI |
1S/C19H24N2O2/c22-18-13-20(19(23)15-7-2-1-3-8-15)12-17-16-9-5-4-6-14(16)10-11-21(17)18/h4-6,9,15,17H,1-3,7-8,10-13H2
|
|||
| InChIKey |
FSVJFNAIGNNGKK-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 55268-74-1
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9571, 603043, 855582, 858012, 4500852, 7847537, 7980375, 8149489, 8153009, 10321427, 10589244, 11335305, 11360544, 11364013, 11366575, 11369137, 11371799, 11374102, 11377299, 11461516, 11466288, 11467408, 11485031, 11486035, 11489104, 11490366, 11492297, 11494933, 12015327, 15272465, 17405467, 22389787, 24278635, 24870350, 26611882, 26680161, 26747203, 26747204, 29223969, 46507082, 47217033, 47291354, 47589213, 47662536, 48035386, 48185224, 48259483, 48259484, 48334763, 48414364
|
|||
| ChEBI ID |
CHEBI:91583
|
|||
| ADReCS Drug ID | BADD_D01826 | |||
| SuperDrug ATC ID |
P02BA01
|
|||
| SuperDrug CAS ID |
cas=055268741
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Glutathione-dependent PGD synthase (HPGDS) | Target Info | Inhibitor | [2] |
| BioCyc | C20 prostanoid biosynthesis | |||
| KEGG Pathway | Arachidonic acid metabolism | |||
| Metabolic pathways | ||||
| WikiPathways | Arachidonic acid metabolism | |||
| Aryl Hydrocarbon Receptor | ||||
| Integrated Pancreatic Cancer Pathway | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Opportunities and challenges in antiparasitic drug discovery. Nat Rev Drug Discov. 2005 Sep;4(9):727-40. | |||
| REF 2 | X-ray structure of glutathione S-transferase from Schistosoma japonicum in a new crystal form reveals flexibility of the substrate-binding site. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2005 Mar 1;61(Pt 3):263-5. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

