Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0LI1C
|
|||
| Former ID |
DNC011182
|
|||
| Drug Name |
NITD609
|
|||
| Synonyms |
Cipargamin; 1193314-23-6; NITD-609; NITD 609; UNII-Z7Q4FWA04P; (1'R,3'S)-5,7'-Dichloro-6'-fluoro-3'-methyl-2',3',4',9'-tetrahydrospiro[indoline-3,1'-pyrido[3,4-b]indol]-2-one; Z7Q4FWA04P; CHEMBL1082723; Spiro[3H-indole-3,1'-[1H]pyrido[3,4-b]indol]-2(1H)-one, 5,7'-dichloro-6'-fluoro-2',3',4',9'-tetrahydro-3'-methyl-, (1'R,3'S)-; Cipargamin [INN]; 1252008-89-1; GTPL9721; SCHEMBL1306342; DTXSID70152424; MolPort-039-337-269; ZINC49037032; BDBM50318666; AKOS027253851; SB16518; DB12306; CS-7486; NCGC00263785-01
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Malaria [ICD-11: 1F40-1F45; ICD-10: B50-B64, B54] | Phase 2 | [1] | |
| Structure |
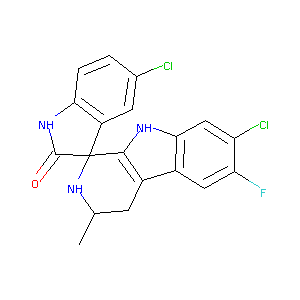 |
Download2D MOL |
||
| Formula |
C19H14Cl2FN3O
|
|||
| Canonical SMILES |
CC1CC2=C(C3(N1)C4=C(C=CC(=C4)Cl)NC3=O)NC5=CC(=C(C=C25)F)Cl
|
|||
| InChI |
1S/C19H14Cl2FN3O/c1-8-4-11-10-6-14(22)13(21)7-16(10)23-17(11)19(25-8)12-5-9(20)2-3-15(12)24-18(19)26/h2-3,5-8,23,25H,4H2,1H3,(H,24,26)/t8-,19+/m0/s1
|
|||
| InChIKey |
CKLPLPZSUQEDRT-WPCRTTGESA-N
|
|||
| CAS Number |
CAS 1193314-23-6
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Adenosine A3 receptor (ADORA3) | Target Info | Inhibitor | [2] |
| Voltage-gated potassium channel Kv11.1 (KCNH2) | Target Info | Inhibitor | [2] | |
| Pathwhiz Pathway | Muscle/Heart Contraction | |||
| Reactome | Adenosine P1 receptors | |||
| G alpha (i) signalling events | ||||
| Voltage gated Potassium channels | ||||
| WikiPathways | Nucleotide GPCRs | |||
| GPCRs, Class A Rhodopsin-like | ||||
| GPCRs, Other | ||||
| SIDS Susceptibility Pathways | ||||
| Hematopoietic Stem Cell Differentiation | ||||
| Potassium Channels | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT01836458) A Study to Find the Minimum Inhibitory Concentration of KAE609 in Adult Male Patients With P. Falciparum Monoinfection. U.S. National Institutes of Health. | |||
| REF 2 | Spiroindolones, a potent compound class for the treatment of malaria. Science. 2010 Sep 3;329(5996):1175-80. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

