Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0M3IN
|
|||
| Former ID |
DCL000293
|
|||
| Drug Name |
Xanthine
|
|||
| Synonyms |
xanthine; 69-89-6; 2,6-Dihydroxypurine; Xanthin; 2,6-dioxopurine; Pseudoxanthine; Isoxanthine; 1H-Purine-2,6(3H,7H)-dione; Xanthic oxide; 1H-Purine-2,6-diol; 9H-Purine-2,6-diol; Purine-2,6-diol; 3,7-Dihydro-1H-purine-2,6-dione; 2,6(1,3)-Purinedion; 1H-Purine-2,6-dione, 3,7-dihydro-; 3,7-dihydropurine-2,6-dione; USAF CB-17; XAN; Purine-2(3H),6(1H)-dione; 2,6-Dioxo-1,2,3,6-tetrahydropurine; 3,9-dihydro-1H-purine-2,6-dione; 9H-Purine-2,6-(1H,3H)-dione; 1H-Purine-2,6-dione, 3,9-dihydro-; Purine-2,6-(1H,3H)-dione; 9H-xanthine
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Apnea [ICD-11: MD11.0; ICD-9: 786.03] | Phase 1 | [1], [2] | |
| Company |
Schering-Plough
|
|||
| Structure |
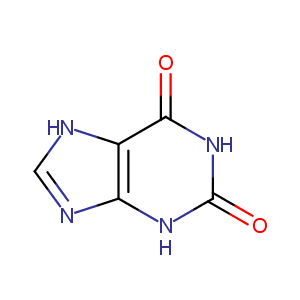 |
Download2D MOL |
||
| Formula |
C5H4N4O2
|
|||
| Canonical SMILES |
C1=NC2=C(N1)C(=O)NC(=O)N2
|
|||
| InChI |
1S/C5H4N4O2/c10-4-2-3(7-1-6-2)8-5(11)9-4/h1H,(H3,6,7,8,9,10,11)
|
|||
| InChIKey |
LRFVTYWOQMYALW-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 69-89-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
3286, 3675, 540766, 618939, 640647, 819059, 855329, 3135149, 3293457, 5455854, 6505008, 6533214, 7891115, 8144038, 8151018, 10533229, 11536787, 15120174, 15946662, 16142320, 16275634, 21317893, 24439533, 24771321, 24902122, 24902144, 24902156, 26683877, 26757757, 26988539, 31462592, 37613684, 38552853, 46507575, 47178680, 48036254, 48111548, 49658808, 49737749, 49748678, 50063430, 56435543, 56465164, 57320802, 58686048, 76273918, 81041251, 81054343, 85090415, 85164959
|
|||
| ChEBI ID |
CHEBI:17712
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Adenosine A2b receptor (ADORA2B) | Target Info | Antagonist | [3] |
| KEGG Pathway | Rap1 signaling pathway | |||
| Calcium signaling pathway | ||||
| Neuroactive ligand-receptor interaction | ||||
| Vascular smooth muscle contraction | ||||
| Alcoholism | ||||
| NetPath Pathway | TGF_beta_Receptor Signaling Pathway | |||
| TCR Signaling Pathway | ||||
| Pathwhiz Pathway | Intracellular Signalling Through Adenosine Receptor A2b and Adenosine | |||
| Pathway Interaction Database | C-MYB transcription factor network | |||
| Reactome | Adenosine P1 receptors | |||
| G alpha (s) signalling events | ||||
| Surfactant metabolism | ||||
| WikiPathways | Nucleotide GPCRs | |||
| GPCRs, Class A Rhodopsin-like | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4557). | |||
| REF 2 | Febuxostat (TMX-67), a novel, non-purine, selective inhibitor of xanthine oxidase, is safe and decreases serum urate in healthy volunteers. Nucleosides Nucleotides Nucleic Acids. 2004 Oct;23(8-9):1111-6. | |||
| REF 3 | Role of adenosine in asthma. Drug Dev Res. 1996;39:333-6. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

