Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0M6BD
|
|||
| Former ID |
DIB002388
|
|||
| Drug Name |
RO-5126766
|
|||
| Synonyms |
VS-6766; CH-5126766; Dual Raf/MEK protein kinase inhibitor (cancer), Roche
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Non-small-cell lung cancer [ICD-11: 2C25] | Phase 2 | [1] | |
| Ovarian cancer [ICD-11: 2C73; ICD-10: C56; ICD-9: 183] | Phase 2 | [1] | ||
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C76-C80; ICD-9: 140-229] | Phase 1 | [2] | ||
| Company |
Roche; Verastem Oncology
|
|||
| Structure |
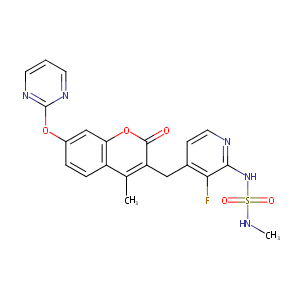 |
Download2D MOL |
||
| Formula |
C21H18FN5O5S
|
|||
| Canonical SMILES |
CC1=C(C(=O)OC2=C1C=CC(=C2)OC3=NC=CC=N3)CC4=C(C(=NC=C4)NS(=O)(=O)NC)F
|
|||
| InChI |
1S/C21H18FN5O5S/c1-12-15-5-4-14(31-21-25-7-3-8-26-21)11-17(15)32-20(28)16(12)10-13-6-9-24-19(18(13)22)27-33(29,30)23-2/h3-9,11,23H,10H2,1-2H3,(H,24,27)
|
|||
| InChIKey |
LMMJFBMMJUMSJS-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 946128-88-7
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:78825
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04625270) A Study of VS-6766 v. VS-6766 + Defactinib in Recurrent Low-Grade Serous Ovarian Cancer With and Without a KRAS Mutation. U.S. National Institutes of Health. | |||
| REF 2 | ClinicalTrials.gov (NCT02407509) Phase I Trial of RO5126766. U.S. National Institutes of Health. | |||
| REF 3 | The dual RAF/MEK inhibitor CH5126766/RO5126766 may be a potential therapy for RAS-mutated tumor cells.PLoS One.2014 Nov 25;9(11):e113217. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

