Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0MT1Q
|
|||
| Former ID |
DIB003155
|
|||
| Drug Name |
K-201
|
|||
| Synonyms |
DKKLXCRMAXNIJF-UHFFFAOYSA-N; UNII-0I621Y6R4Q; K201; 0I621Y6R4Q; 1038410-88-6; K 201; SCHEMBL194018; CHEMBL2440857; DTXSID90146108; K-201, Moberg; Dermatological drug combination (kaprolac, atopic dermatitis), Moberg; Urea + PEG/propylene glycol formulation (atopic dermatitis), Moberg; JTV 519
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Atopic dermatitis [ICD-11: EA80; ICD-10: L20; ICD-9: 691.8, 692.9] | Phase 2 | [1] | |
| Company |
Moberg Derma AB
|
|||
| Structure |
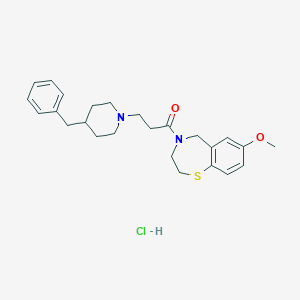 |
Download2D MOL
|
||
| Formula |
C25H33ClN2O2S
|
|||
| Canonical SMILES |
COC1=CC2=C(C=C1)SCCN(C2)C(=O)CCN3CCC(CC3)CC4=CC=CC=C4.Cl
|
|||
| InChI |
1S/C25H32N2O2S.ClH/c1-29-23-7-8-24-22(18-23)19-27(15-16-30-24)25(28)11-14-26-12-9-21(10-13-26)17-20-5-3-2-4-6-20;/h2-8,18,21H,9-17,19H2,1H3;1H
|
|||
| InChIKey |
DKKLXCRMAXNIJF-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1038410-88-6
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Potassium channel unspecific (KC) | Target Info | Modulator | [2], [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT01067833) Phase 2 Study of Oral K201 for Prevention of AF Recurrence. U.S. National Institutes of Health. | |||
| REF 2 | Cardiac ryanodine receptor in metabolic syndrome: is JTV519 (K201) future therapy | |||
| REF 3 | JTV519 (K201) reduces sarcoplasmic reticulum Ca leak and improves diastolic function in vitro in murine and human non-failing myocardium. Br J Pharmacol.2012 Oct;167(3):493-504. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

