Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0MW7B
|
|||
| Former ID |
DIB008971
|
|||
| Drug Name |
PF-4494700
|
|||
| Synonyms |
PF-04494700; TTP-488; TTP-488); Alzheimers therapy, TransTech/Pfizer; Diabetic nephropathy therapy, Transtech/Pfizer; Alzheimer'streatment, TransTech/Pfizer; Alzheimer's therapy (RAGE), Transtech/Pfizer
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Alzheimer disease [ICD-11: 8A20; ICD-10: G30, G30.9; ICD-9: 331] | Phase 3 | [1], [2] | |
| Dementia [ICD-11: 6D80-6D86] | Phase 3 | [3], [4] | ||
| Company |
Transtech pharma
|
|||
| Structure |
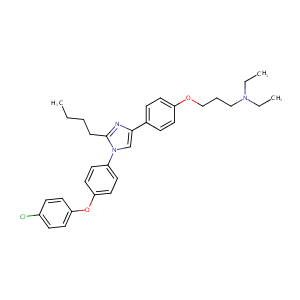 |
Download2D MOL
|
||
| Formula |
C32H38ClN3O2
|
|||
| Canonical SMILES |
CCCCC1=NC(=CN1C2=CC=C(C=C2)OC3=CC=C(C=C3)Cl)C4=CC=C(C=C4)OCCCN(CC)CC
|
|||
| InChI |
1S/C32H38ClN3O2/c1-4-7-9-32-34-31(25-10-16-28(17-11-25)37-23-8-22-35(5-2)6-3)24-36(32)27-14-20-30(21-15-27)38-29-18-12-26(33)13-19-29/h10-21,24H,4-9,22-23H2,1-3H3
|
|||
| InChIKey |
KJNNWYBAOPXVJY-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 603148-36-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Advanced glycosylation end product receptor (AGER) | Target Info | Antagonist | [5] |
| Pathway Interaction Database | amb2 Integrin signaling | |||
| Reactome | RIP-mediated NFkB activation via ZBP1 | |||
| DEx/H-box helicases activate type I IFN and inflammatory cytokines production | ||||
| TAK1 activates NFkB by phosphorylation and activation of IKKs complex | ||||
| Advanced glycosylation endproduct receptor signaling | ||||
| TRAF6 mediated NF-kB activation | ||||
| WikiPathways | NRF2 pathway | |||
| Nuclear Receptors Meta-Pathway | ||||
| Cytosolic sensors of pathogen-associated DNA | ||||
| TAK1 activates NFkB by phosphorylation and activation of IKKs complex | ||||
| AGE/RAGE pathway | ||||
| RIG-I/MDA5 mediated induction of IFN-alpha/beta pathways | ||||
| Advanced glycosylation endproduct receptor signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8317). | |||
| REF 4 | ClinicalTrials.gov (NCT02080364) Evaluation of the Efficacy and Safety of Azeliragon (TTP488) in Patients With Mild Alzheimer's Disease. U.S. National Institutes of Health. | |||
| REF 5 | Receptor for advanced glycation endproduct modulators: a new therapeutic target in Alzheimer's disease. Expert Opin Investig Drugs. 2015 Mar;24(3):393-9. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

