Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0N1TH
|
|||
| Former ID |
DNCL001883
|
|||
| Drug Name |
ATI-2042
|
|||
| Synonyms |
Budiodarone
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Atrial fibrillation [ICD-11: BC81.3; ICD-10: I48] | Phase 2 | [1] | |
| Company |
ARYx Therapeutics
|
|||
| Structure |
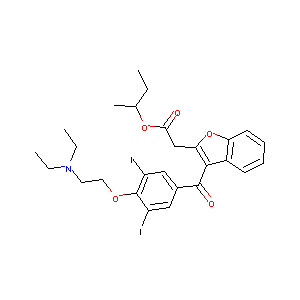 |
Download2D MOL |
||
| Formula |
C27H31I2NO5
|
|||
| Canonical SMILES |
CCC(C)OC(=O)CC1=C(C2=CC=CC=C2O1)C(=O)C3=CC(=C(C(=C3)I)OCCN(CC)CC)I
|
|||
| InChI |
1S/C27H31I2NO5/c1-5-17(4)34-24(31)16-23-25(19-10-8-9-11-22(19)35-23)26(32)18-14-20(28)27(21(29)15-18)33-13-12-30(6-2)7-3/h8-11,14-15,17H,5-7,12-13,16H2,1-4H3/t17-/m0/s1
|
|||
| InChIKey |
ZXOSVKYCXLTVGS-KRWDZBQOSA-N
|
|||
| CAS Number |
CAS 335148-45-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Calcium channel unspecific (CaC) | Target Info | Agonist | [2] |
| Ion channel unspecific (IC) | Target Info | Antagonist | [3] | |
| Potassium channel unspecific (KC) | Target Info | Agonist | [2] | |
| Sodium channel unspecific (NaC) | Target Info | Agonist | [2] | |
| KEGG Pathway | Dopaminergic synapse | |||
| Reactome | Interaction between L1 and Ankyrins | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00389792) Efficacy and Safety Study of an Antiarrhythmic Drug to Treat Atrial Fibrillation in Patients With Pacemakers. U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | A randomized trial of budiodarone in paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2012 Jun;34(1):1-9. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

