Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0N6SO
|
|||
| Former ID |
DCL000647
|
|||
| Drug Name |
SYR-472
|
|||
| Synonyms |
Trelagliptin succinate; TRELAGLIPTIN SUCCINATE; 1029877-94-8; Trelagliptin (succinate); UNII-4118932Z90; AK198895; 2-[[6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxopyrimidin-1-yl]methyl]-4-fluorobenzonitrile; 4118932Z90; SYR-472 succinate; (R)-2-((6-(3-aminopiperidin-1-yl)-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)methyl)-4-fluorobenzonitrile succinate; SYR 111472 succinate; Trelagliptin succinate [USAN]; SYR111472 SUCCINATE; SYR 472; SYR472; Zafatek (TN); Trelagliptin succinat; SYR472 succinate; syr; Trelagliptin Succinate
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Metabolic disorder [ICD-11: 5C50-5D2Z; ICD-10: E70-E90; ICD-9: 270-279] | Phase 3 | [1] | |
| Non-insulin dependent diabetes [ICD-11: 5A11] | Investigative | [2] | ||
| Company |
Takeda
|
|||
| Structure |
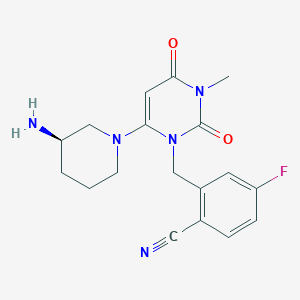 |
Download2D MOL |
||
| Formula |
C18H20FN5O2
|
|||
| Canonical SMILES |
CN1C(=O)C=C(N(C1=O)CC2=C(C=CC(=C2)F)C#N)N3CCCC(C3)N
|
|||
| InChI |
1S/C18H20FN5O2/c1-22-17(25)8-16(23-6-2-3-15(21)11-23)24(18(22)26)10-13-7-14(19)5-4-12(13)9-20/h4-5,7-8,15H,2-3,6,10-11,21H2,1H3/t15-/m1/s1
|
|||
| InChIKey |
IWYJYHUNXVAVAA-OAHLLOKOSA-N
|
|||
| CAS Number |
CAS 865759-25-7
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ChEBI ID |
CHEBI:134715
|
|||
| SuperDrug ATC ID |
R03AC09
|
|||
| SuperDrug CAS ID |
cas=018559596
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Dipeptidyl peptidase 4 (DPP-4) | Target Info | Inhibitor | [2], [3], [4] |
| KEGG Pathway | Protein digestion and absorption | |||
| NetPath Pathway | IL2 Signaling Pathway | |||
| TGF_beta_Receptor Signaling Pathway | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of Takeda. | |||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 1612). | |||
| REF 3 | SYR-472, a novel once-weekly dipeptidyl peptidase-4 (DPP-4) inhibitor, in type 2 diabetes mellitus: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014 Feb;2(2):125-32. | |||
| REF 4 | Clinical pipeline report, company report or official report of Takeda (2009). | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

