Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0O4EU
|
|||
| Former ID |
DAP001343
|
|||
| Drug Name |
Acrivastine
|
|||
| Synonyms |
Acrivastin; Acrivastina; Acrivastinum; Semprex; Acrivastina [Spanish]; Acrivastinum [Latin]; Benadryl allergy relief; BW 0270C; BW 825C; BW A825C; BW-825C; Acrivastine (USAN/INN); Acrivastine [USAN:INN:BAN]; E-(9CI); (2E)-3-{6-[(1E)-1-(4-methylphenyl)-3-pyrrolidin-1-ylprop-1-en-1-yl]pyridin-2-yl}prop-2-enoic acid; (E)-3-[6-[(E)-1-(4-methylphenyl)-3-pyrrolidin-1-ylprop-1-enyl]pyridin-2-yl]prop-2-enoic acid; (E)-6-((E)-3-(1-Pyrrolidinyl)-1-p-tolylpropenyl)-2-pyridineacrylic acid; (E)-6-((E)-3-(1-Pyrrolidinyl-1-p-tolylpropenyl)-2-pyridinacrylsaeure; (E,E)-3-[6-[1-(4-methylphenyl)-3-(1-pyrrolidinyl)-1-propenyl]-2-pyridinyl]-2-propenoic acid; 3-{6-[1-(4-methylphenyl)-3-(pyrrolidin-1-yl)prop-1-en-1-yl]pyridin-2-yl}prop-2-enoic acid
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Allergic rhinitis [ICD-11: CA08.0] | Approved | [1] | |
| Therapeutic Class |
Antiinflammatory Agents
|
|||
| Company |
Pfizer Pharmaceuticals
|
|||
| Structure |
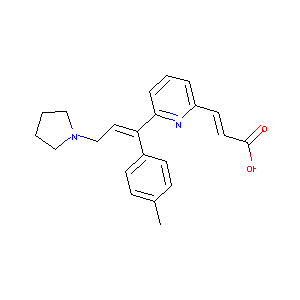 |
Download2D MOL |
||
| Formula |
C22H24N2O2
|
|||
| Canonical SMILES |
CC1=CC=C(C=C1)C(=CCN2CCCC2)C3=CC=CC(=N3)C=CC(=O)O
|
|||
| InChI |
1S/C22H24N2O2/c1-17-7-9-18(10-8-17)20(13-16-24-14-2-3-15-24)21-6-4-5-19(23-21)11-12-22(25)26/h4-13H,2-3,14-16H2,1H3,(H,25,26)/b12-11+,20-13+
|
|||
| InChIKey |
PWACSDKDOHSSQD-IUTFFREVSA-N
|
|||
| CAS Number |
CAS 87848-99-5
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
7978641, 11039320, 11528790, 12012900, 14827611, 17396918, 39317835, 48415515, 50478506, 57359099, 92719198, 92729786, 93166367, 93309492, 99299401, 103374556, 104178336, 109614385, 113863077, 117544906, 124893773, 126628312, 126651909, 126670557, 127327803, 134338557, 135260703, 137100857, 142971128, 144206566, 162180352, 175268536, 179151367, 185967402, 196106033, 223447811, 223683999, 223704756, 226396645, 252344698
|
|||
| ChEBI ID |
CHEBI:83168
|
|||
| SuperDrug ATC ID |
R06AX18
|
|||
| SuperDrug CAS ID |
cas=087848995
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Histamine H1 receptor (H1R) | Target Info | Antagonist | [2], [3] |
| KEGG Pathway | Calcium signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Inflammatory mediator regulation of TRP channels | ||||
| Panther Pathway | Histamine H1 receptor mediated signaling pathway | |||
| Reactome | Histamine receptors | |||
| G alpha (q) signalling events | ||||
| WikiPathways | Monoamine GPCRs | |||
| GPCRs, Class A Rhodopsin-like | ||||
| IL-4 Signaling Pathway | ||||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Comparative tolerability of second generation antihistamines. Drug Saf. 1999 May;20(5):385-401. | |||
| REF 2 | Clinical pharmacology of new histamine H1 receptor antagonists. Clin Pharmacokinet. 1999 May;36(5):329-52. | |||
| REF 3 | Nonsedating histamine H1-receptor antagonists. Clin Pharm. 1989 May;8(5):331-44. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

