Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0OT0O
|
|||
| Former ID |
DAP001383
|
|||
| Drug Name |
Foscavir
|
|||
| Synonyms |
Foscarmet; Foscarnet; Phosphonoformate; Triapten; Virudin; Carboxyphosphonic acid; Dihydroxyphosphanecarboxylic acid oxide; Dihydroxyphosphinecarboxylic acid oxide; Dihydroxyphosphinecarboxylic acid oxide trisodium salt; Dihydroxyphosphinecarboxylic acid oxide trisodium salt hexahydrate; FOSCARNET SODIUM; Forscarnet sodium; Foscarnet sodico; Foscarnet sodico [Spanish]; Foscarnet sodique; Foscarnet sodique [French]; Foscarnet sodium hexahydrate; Foscarnet sodium hydrate; Foscarneto sodico; Foscarnetum natricum; Foscarnetum natricum [Latin]; Phgosphonocarboxylic acid; Phosphonoformic acid; Phosphonomethanoic acid; Trisodium carboxyphosphate; Trisodium dioxidophosphanecarboxylate oxide; Trisodium dioxidophosphinecarboxylate oxide; Trisodium phosphonatoformate; Trisodium phosphonatoformate hexahydrate; Trisodium phosphonoformate; Trisodium phosphonoformate hexahydrate; Trisodium phosphonoformte hexahydrate; A 29622; EHB 776; A-29622; DRG-0017; Dihydroxyphosphinecarboxylic acid oxide and MSL, neutralizing monoclonal antibody; EHB-776; Foscarnet sodique [INN-French]; Foscarnet sodium hydrate (JAN); Foscarneto sodico [INN-Spanish]; Foscarnetum natricum [INN-Latin]; Foscavir (TN); HS-0008; MSL & PFA; Phosphonoformic acid, trisodium salt; Trisodium carboxyphosphate (anhydrous); Trisodium phosphonoformate (anhydrous); Foscarnet & IFN-ALPHA; Foscarnet sodium (USAN/INN); Foscarnet sodium [USAN:INN:BAN]; Phosphonoformic acid & IFN-ALPHA; Phosphonoformic acid, trisodium salt, hexahydrate; PFA & rIFN.alpha.A; Phosphinecarboxylic acid, dihydroxy-, oxide; Phosphonoformate(trisodium) & Recombinant Alpha-A Interferon; Sodium dioxidophosphanecarboxylate oxide hydrate(3:1:6); Formic acid, phosphono-, trisodium salt, hexahydrate; Phosphinecarboxylic acid, dihydroxy-, oxide, trisodium salt; Phosphinecarboxylic acid, dihydroxy-, oxide, trisodium salt, hexahydrate
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Cytomegalovirus retinitis [ICD-11: 9B72.00; ICD-9: 78.5] | Approved | [1] | |
| Therapeutic Class |
Antiviral Agents
|
|||
| Company |
AstraZeneca
|
|||
| Structure |
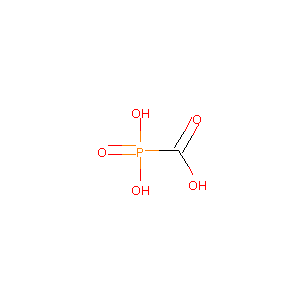 |
Download2D MOL |
||
| Formula |
CNa3O5P
|
|||
| Canonical SMILES |
C(=O)([O-])P(=O)([O-])[O-].[Na+].[Na+].[Na+]
|
|||
| InChI |
1S/CH3O5P.3Na/c2-1(3)7(4,5)6;;;/h(H,2,3)(H2,4,5,6);;;/q;3*+1/p-3
|
|||
| InChIKey |
DFHAXXVZCFXGOQ-UHFFFAOYSA-K
|
|||
| CAS Number |
CAS 63585-09-1
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
7847645, 8149794, 8178763, 10196943, 12012803, 14772938, 26612258, 26679458, 26748573, 26748574, 34709806, 48416037, 50006945, 50534151, 57312956, 57357103, 85341314, 91010671, 92124322, 92307785, 92714305, 99302003, 103202136, 104345184, 108146945, 113004708, 119526189, 124637846, 125312361, 125537224, 126622014, 126624248, 126645593, 126655507, 126669585, 135006490, 137267469, 144204917, 152090806, 160843930, 162177321, 162263575, 163134832, 164810490, 170465060, 179210376, 184545040, 196106557, 198969523, 223677244
|
|||
| ChEBI ID |
CHEBI:141644
|
|||
| ADReCS Drug ID | BADD_D00964 ; BADD_D02421 | |||
| SuperDrug ATC ID |
J05AD01
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Herpes simplex virus DNA polymerase UL30 (HSV UL30) | Target Info | Inhibitor | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 020068. | |||
| REF 2 | Drug targets in cytomegalovirus infection. Infect Disord Drug Targets. 2009 Apr;9(2):201-22. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

