Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0P1YE
|
|||
| Former ID |
DCL000585
|
|||
| Drug Name |
Orvepitant
|
|||
| Synonyms |
Orvepitant; UNII-IIU6V0W3JD; 579475-18-6; GW823296X; IIU6V0W3JD; GW823296; Orvepitant [USAN:INN]; Orvepitant (USAN/INN); SCHEMBL1421784; CHEMBL2105667; XWNBGDJPEXZSQM-VZOBGQTKSA-N; BDBM50442585; ZINC56898864; AKOS030231272; SB17117; DB12427; NCGC00386593-01; (2R,4S)-N-((R)-1-(3,5-Bis(trifluoromethyl)phenyl)ethyl)-2-(4-fluoro-2-methylphenyl)-N-methyl-4-((S)-6-oxohexahydropyrrolo[1,2-a]pyrazin-2(1H)-yl)piperidine-1-c; D09650
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Anxiety disorder [ICD-11: 6B00-6B0Z] | Phase 2 | [1] | |
| Depression [ICD-11: 6A70-6A7Z] | Phase 2 | [1] | ||
| Company |
GSK
|
|||
| Structure |
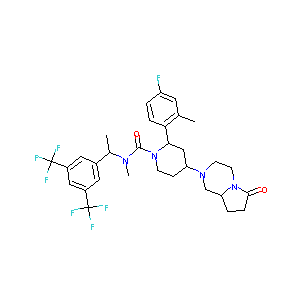 |
Download2D MOL |
||
| Formula |
C31H35F7N4O2
|
|||
| Canonical SMILES |
CC1=C(C=CC(=C1)F)C2CC(CCN2C(=O)N(C)C(C)C3=CC(=CC(=C3)C(F)(F)F)C(F)(F)F)N4CCN5C(C4)CCC5=O
|
|||
| InChI |
1S/C31H35F7N4O2/c1-18-12-23(32)4-6-26(18)27-16-24(40-10-11-41-25(17-40)5-7-28(41)43)8-9-42(27)29(44)39(3)19(2)20-13-21(30(33,34)35)15-22(14-20)31(36,37)38/h4,6,12-15,19,24-25,27H,5,7-11,16-17H2,1-3H3/t19-,24+,25+,27-/m1/s1
|
|||
| InChIKey |
XWNBGDJPEXZSQM-VZOBGQTKSA-N
|
|||
| CAS Number |
CAS 579475-18-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Substance-P receptor (TACR1) | Target Info | Antagonist | [2] |
| KEGG Pathway | Calcium signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Measles | ||||
| Panther Pathway | CCKR signaling map ST | |||
| Reactome | G alpha (q) signalling events | |||
| WikiPathways | SIDS Susceptibility Pathways | |||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| Spinal Cord Injury | ||||
| Peptide GPCRs | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800020168) | |||
| REF 2 | Clinical pipeline report, company report or official report of GlaxoSmithKline (2009). | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

