Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0P2GK
|
|||
| Former ID |
DIB012588
|
|||
| Drug Name |
Sodium phenylbutyrate
|
|||
| Synonyms |
Ammonaps; Buphenyl; EL-532; Sodium 4-phenylbutyrate; Sodium phenylbutyrate, Elan; Sodium phenylbutyrate, Medicis; Sodium phenylbutyrate, Ucyclyd; VP-101; Sodium 4-phenylbutyrate, Elan; Sodium 4-phenylbutyrate, Ucyclyd; Sodium phenylbutyrate (cancer), MacroChem/Access; Sodium phenylbutyrate (cancer), Virium/ Somanta; Sodium phenylbutyrate (cancer), Virium/Access Pharmaceuticals; Sodium 4-phenyibutyrate (cancer), MacroChem/Access; Sodium 4-phenylbutyrate (cancer), Virium/ Somanta; Sodium 4-phenylbutyrate (cancer), Virium/Access Pharmaceuticals
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Spinal muscular atrophy [ICD-11: 8B61] | Approved | [1] | |
| Company |
Ucyclyd Pharma Inc; Elan Corp plc
|
|||
| Structure |
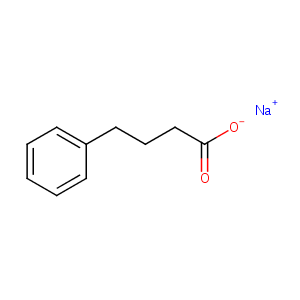 |
Download2D MOL |
||
| Formula |
C10H11NaO2
|
|||
| Canonical SMILES |
C1=CC=C(C=C1)CCCC(=O)[O-].[Na+]
|
|||
| InChI |
1S/C10H12O2.Na/c11-10(12)8-4-7-9-5-2-1-3-6-9;/h1-3,5-6H,4,7-8H2,(H,11,12);/q;+1/p-1
|
|||
| InChIKey |
VPZRWNZGLKXFOE-UHFFFAOYSA-M
|
|||
| CAS Number |
CAS 1716-12-7
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:75316
|
|||
| ADReCS Drug ID | BADD_D01760 ; BADD_D02048 | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00533559) Mechanism of Fatty Acid-induced Impairment of Glucose-simulated Insulin Secretion - Effect of Buphenyl. U.S. National Institutes of Health. | |||
| REF 2 | Histone Deacetylase inhibitors: new promise in the treatment of immune and inflammatory diseases. Curr Drug Targets. 2010 Nov;11(11):1430-8. | |||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 4 | Polo-like kinase inhibitor Ro5203280 has potent antitumor activity in nasopharyngeal carcinoma.Mol Cancer Ther. 2013 Aug;12(8):1393-401. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

