Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0P2IW
|
|||
| Former ID |
DCL000286
|
|||
| Drug Name |
Peramivir
|
|||
| Synonyms |
PeramiFlu; S-021812; (1S,2S,3S,4R)-3-(1-acetamido-2-ethylbutyl)-4-(diaminomethylideneamino)-2-hydroxycyclopentane-1-carboxylic acid; (1S,2S,3S,4R)-3-[(1S)-1-acetamido-2-ethylbutyl]-4-(diaminomethylideneamino)-2-hydroxycyclopentane-1-carboxylic acid; PRV
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Influenza virus infection [ICD-11: 1E30-1E32] | Approved | [1] | |
| Company |
BioCryst Pharmaceuticals
|
|||
| Structure |
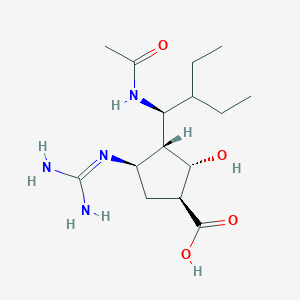 |
Download2D MOL |
||
| Formula |
C15H28N4O4
|
|||
| Canonical SMILES |
CCC(CC)C(C1C(CC(C1O)C(=O)O)N=C(N)N)NC(=O)C
|
|||
| InChI |
1S/C15H28N4O4/c1-4-8(5-2)12(18-7(3)20)11-10(19-15(16)17)6-9(13(11)21)14(22)23/h8-13,21H,4-6H2,1-3H3,(H,18,20)(H,22,23)(H4,16,17,19)/t9-,10+,11+,12-,13+/m0/s1
|
|||
| InChIKey |
XRQDFNLINLXZLB-CKIKVBCHSA-N
|
|||
| CAS Number |
CAS 330600-85-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ChEBI ID |
CHEBI:85202
|
|||
| ADReCS Drug ID | BADD_D01732 | |||
| Drug Resistance Mutation (DRM) | Top | |||
|---|---|---|---|---|
| DRM | DRM Info | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Influenza Neuraminidase (Influ NA) | Target Info | Inhibitor | [2], [3] |
| KEGG Pathway | Other glycan degradation | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | 2014 FDA drug approvals. Nat Rev Drug Discov. 2015 Feb;14(2):77-81. | |||
| REF 2 | Developing new antiviral agents for influenza treatment: what does the future hold Clin Infect Dis. 2009 Jan 1;48 Suppl 1:S3-13. | |||
| REF 3 | Current and future antiviral therapy of severe seasonal and avian influenza. Antiviral Res. 2008 Apr;78(1):91-102. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

