Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0Q2XF
|
|||
| Former ID |
DIB007266
|
|||
| Drug Name |
Bimatoprost
|
|||
| Synonyms |
Bimatoprost (topical, alopecia)
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Alopecia [ICD-11: ED70; ICD-9: 704.09] | Approved | [1], [2] | |
| Glaucoma/ocular hypertension [ICD-11: 9C61; ICD-9: 365] | Phase 3 | [3] | ||
| Company |
Allergan Inc
|
|||
| Structure |
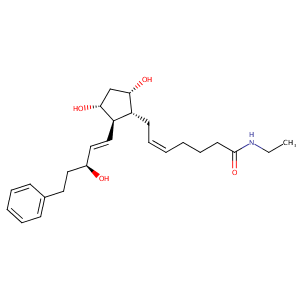 |
Download2D MOL |
||
| Formula |
C25H37NO4
|
|||
| Canonical SMILES |
CCNC(=O)CCCC=CCC1C(CC(C1C=CC(CCC2=CC=CC=C2)O)O)O
|
|||
| InChI |
1S/C25H37NO4/c1-2-26-25(30)13-9-4-3-8-12-21-22(24(29)18-23(21)28)17-16-20(27)15-14-19-10-6-5-7-11-19/h3,5-8,10-11,16-17,20-24,27-29H,2,4,9,12-15,18H2,1H3,(H,26,30)/b8-3-,17-16+/t20-,21+,22+,23-,24+/m0/s1
|
|||
| InChIKey |
AQOKCDNYWBIDND-FTOWTWDKSA-N
|
|||
| CAS Number |
CAS 155206-00-1
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
7978793, 11056218, 11528907, 12015127, 14855657, 14880018, 17396891, 17403938, 39340757, 46505334, 50126297, 50464908, 56464316, 71851420, 90342416, 90342418, 99443334, 103770946, 114155088, 126592972, 126666993, 134223006, 134338457, 135256698, 135649995, 137005798, 142063913, 152134985, 152344136, 160964244, 162180462, 174548912, 175266561, 175437834, 179150000, 210279322, 210281645, 223392784, 223660297, 223704349, 224493205, 226412773, 250184579, 251912536, 251915515, 252214992, 252448483, 252822160
|
|||
| ChEBI ID |
CHEBI:51230
|
|||
| ADReCS Drug ID | BADD_D00274 | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Prostaglandin receptor (PTGR) | Target Info | Modulator | [4] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1958). | |||
| REF 2 | ClinicalTrials.gov (NCT01387906) Latisse (Bimatoprost .03% Opthalmic Solution) for the Treatment of Hypotrichosis of the Eyebrows: Latisse Versus Placebo. U.S. National Institutes of Health. | |||
| REF 3 | ClinicalTrials.gov (NCT02250651) Safety and Efficacy of Bimatoprost Sustained-Release (SR) in Patients With Open-Angle Glaucoma or Ocular Hypertension. U.S. National Institutes of Health. | |||
| REF 4 | Bimatoprost-induced calcium signaling in human T-cells does not involve prostanoid FP or TP receptors. Curr Eye Res. 2009 Mar;34(3):184-95. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

