Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0QF4Z
|
|||
| Former ID |
DNC000965
|
|||
| Drug Name |
MT-500
|
|||
| Synonyms |
199864-87-4; RS-127445; 4-(4-fluoronaphthalen-1-yl)-6-isopropylpyrimidin-2-amine; RS 127445; UNII-0JAU3P8OBM; MT 500; 0JAU3P8OBM; CHEMBL473186; C17H16FN3; RS-127445 HCl; 2-Amino-4-(4-fluoronaphth-1-yl)-6-isopropylpyrimidine; RS127445; 2-Pyrimidinamine, 4-(4-fluoro-1-naphthalenyl)-6-(1-methylethyl)-; RS-127,445; 4-(4-fluoronaphthalen-1-yl)-6-propan-2-ylpyrimidin-2-amine; AC1L52MZ; GTPL188; RS 127445 hydrochloride/; SCHEMBL375979; MolPort-021-804-999; ZZZQXCUPAJFVBN-UHFFFAOYSA-N; HMS3651H11; BCPP000085; BCP02714; ZINC3961115
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Migraine [ICD-11: 8A80; ICD-10: G43, G43.9; ICD-9: 346] | Discontinued in Phase 1 | [1] | |
| Structure |
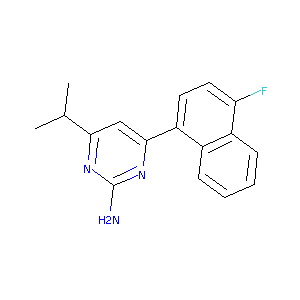 |
Download2D MOL |
||
| Formula |
C17H16FN3
|
|||
| Canonical SMILES |
CC(C)C1=NC(=NC(=C1)C2=CC=C(C3=CC=CC=C32)F)N
|
|||
| InChI |
1S/C17H16FN3/c1-10(2)15-9-16(21-17(19)20-15)13-7-8-14(18)12-6-4-3-5-11(12)13/h3-10H,1-2H3,(H2,19,20,21)
|
|||
| InChIKey |
ZZZQXCUPAJFVBN-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 199864-87-4
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
10262227, 15222255, 33516960, 57398722, 79504188, 85209272, 103595253, 104103501, 113464172, 126453576, 129979478, 135174031, 135650939, 136348836, 136349561, 136367692, 136367798, 137010099, 152344474, 162038151, 162202799, 163404105, 163846793, 164023447, 164764908, 171578608, 179294165, 223384158, 223556713, 225046137, 226707491, 241179982, 242059982, 249825890, 250214735, 251971191, 252061746, 252216232, 252434548, 252447189
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | 5-HT 2B receptor (HTR2B) | Target Info | Antagonist | [2] |
| KEGG Pathway | Calcium signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Gap junction | ||||
| Serotonergic synapse | ||||
| Inflammatory mediator regulation of TRP channels | ||||
| Panther Pathway | 5HT2 type receptor mediated signaling pathway | |||
| Reactome | Serotonin receptors | |||
| G alpha (q) signalling events | ||||
| WikiPathways | Serotonin Receptor 2 and ELK-SRF/GATA4 signaling | |||
| Monoamine GPCRs | ||||
| GPCRs, Class A Rhodopsin-like | ||||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800013923) | |||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

