Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0QNS3
|
|||
| Drug Name |
REL-1017
|
|||
| Synonyms |
Dextromethadone; d-Methadone; S-Methadone; (S)-methadone; (+)-Methadone; S-(+)-Methadone; 6S-Methadone; 5653-80-5; L-(+)-Methadone; (6S)-Methadone; l-Methadone; (S)-(+)-Methadone; UNII-S95RZH8AMH; d-6-(Dimethylamino)-4,4-diphenyl-3-heptanone; S95RZH8AMH; (S)-6-(Dimethylamino)-4,4-diphenyl-3-heptanone; CHEMBL350719; (S)-6-(dimethylamino)-4,4-diphenylheptan-3-one; 3-14-00-00279 (Beilstein Handbook Reference); Methadone, (+)-; BRN 3213667; (+)-(S)-Methadone; (+)-(6S)-Methadone; SCHEMBL24294; (6S)-6-(dimethylamino)-4,4-diphenylheptan-3-one; CHEBI:167308; 3-HEPTANONE, 6-(DIMETHYLAMINO)-4,4-DIPHENYL-, (S)-; ZINC1530707; BDBM50223640; DB15198; SB18896; (S)-6-Dimethylamino4,4-diphenylheptan-3-on; Q15634047; (+)-(S)-6-(dimethylamino)-4,4-diphenyl-3-heptanone; 3-heptanone, 6-(dimethylamino)-4,4-diphenyl-, (6S)-; UNII-UC6VBE7V1Z component USSIQXCVUWKGNF-KRWDZBQOSA-N; UNII-UC6VBE7V1Z component USSIQXCVUWKGNF-QGZVFWFLSA-N; 3-Heptanone, 6-(dimethylamino)-4,4-diphenyl-, (6S)- (9CI); 3-Heptanone, 6-(dimethylamino)-4,4-diphenyl-, (S)- (8CI)
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Major depressive disorder [ICD-11: 6A70.3; ICD-10: F32.2; ICD-9: 296.2, 296.3] | Phase 3 | [1] | |
| Neuropathic pain [ICD-11: 8E43.0] | Phase 1 | [2] | ||
| Company |
Relmada Therapeutics
|
|||
| Structure |
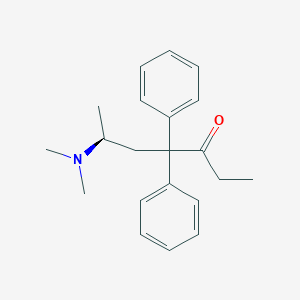 |
Download2D MOL |
||
| Formula |
C21H27NO
|
|||
| Canonical SMILES |
CCC(=O)C(CC(C)N(C)C)(C1=CC=CC=C1)C2=CC=CC=C2
|
|||
| InChI |
1S/C21H27NO/c1-5-20(23)21(16-17(2)22(3)4,18-12-8-6-9-13-18)19-14-10-7-11-15-19/h6-15,17H,5,16H2,1-4H3/t17-/m0/s1
|
|||
| InChIKey |
USSIQXCVUWKGNF-KRWDZBQOSA-N
|
|||
| CAS Number |
CAS 5653-80-5
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:167308
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | N-methyl-D-aspartate receptor (NMDAR) | Target Info | Antagonist | [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04855760) Open Label Study to Assess the Safety of REL-1017 for Major Depressive Disorder (RELIANCE-OLS). U.S. National Institutes of Health. | |||
| REF 2 | ClinicalTrials.gov (NCT03638869) Safety, Tolerability, and Pharmacokinetics of Multiple Ascending Doses of REL-1017 (d-Methadone). U.S. National Institutes of Health. | |||
| REF 3 | N-Methyl-D-aspartate receptor antagonist d-methadone produces rapid, mTORC1-dependent antidepressant effects. Neuropsychopharmacology. 2019 Dec;44(13):2230-2238. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

