Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0QR3X
|
|||
| Drug Name |
Edasalonexent
|
|||
| Synonyms |
UNII-AF3Z6434KS; AF3Z6434KS; 1204317-86-1; Edasalonexent [INN]; SCHEMBL1823117; CHEMBL3786673; JQLBBYLGWHUHRW-KUBAVDMBSA-N; N-(2-(4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenamidoethyl)-2-hydroxybenzamide; Benzamide, 2-hydroxy-N-(2-(((4Z,7Z,10Z,13Z,16Z,19Z)-1-oxo-4,7,10,13,16,19-docosahexaen-1-yl)amino)ethyl)-; N-(2-(4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16, 19-hexaenamidoethyl)-2-hydroxybenzamide
Click to Show/Hide
|
|||
| Indication | Duchenne dystrophy [ICD-11: 8C70; ICD-10: G71.0] | Phase 3 | [1] | |
| Company |
Catabasis Pharmaceuticals Cambridge, MA
|
|||
| Structure |
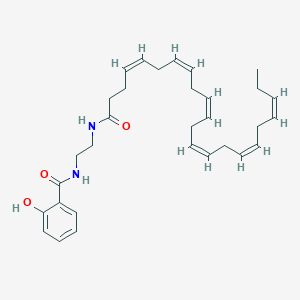 |
Download2D MOL |
||
| Formula |
C31H42N2O3
|
|||
| Canonical SMILES |
CCC=CCC=CCC=CCC=CCC=CCC=CCCC(=O)NCCNC(=O)C1=CC=CC=C1O
|
|||
| InChI |
1S/C31H42N2O3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-25-30(35)32-26-27-33-31(36)28-23-21-22-24-29(28)34/h3-4,6-7,9-10,12-13,15-16,18-19,21-24,34H,2,5,8,11,14,17,20,25-27H2,1H3,(H,32,35)(H,33,36)/b4-3-,7-6-,10-9-,13-12-,16-15-,19-18-
|
|||
| InChIKey |
JQLBBYLGWHUHRW-KUBAVDMBSA-N
|
|||
| CAS Number |
CAS 1204317-86-1
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03917719) An Open-Label Extension Study of Edasalonexent in Boys With Duchenne Muscular Dystrophy (GalaxyDMD). U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

