Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0R7QY
|
|||
| Former ID |
DNC003634
|
|||
| Drug Name |
EMATE
|
|||
| Synonyms |
Emate compound; Estrone 3-O-Sulfamate; EMATE; Estrone-3-O-sulfamate; Oestrone-3-O-sulphamate; 148672-09-7; Estra-1,3,5(10)-trien-17-one-3-sulfamate; CHEMBL122708; 3-((Aminosulfonyl)oxy)estra-1,3,5(10)-trien-17-one; C18H23NO4S; 3-[(Aminosulfonyl)oxy]estra-1,3,5(10)-trien-17-one; Estra-1,3,5(10)-trien-17-one, 3-((aminosulfonyl)oxy)-; Estrone Sulfamate; estrone3-O-sulfamate; NSC685426; Estrone O-Sulfamate; Estrone 3-Sulfamate; AC1L2SFZ; SCHEMBL305660; CTK8F0296; RVKFQAJIXCZXQY-CBZIJGRNSA-N; ZINC5832614; BDBM50134329; AKOS030241107
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
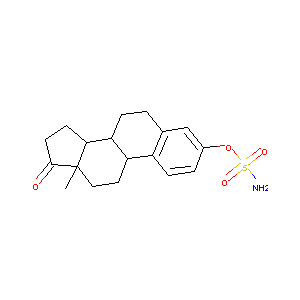 |
Download2D MOL |
||
| Formula |
C18H23NO4S
|
|||
| Canonical SMILES |
CC12CCC3C(C1CCC2=O)CCC4=C3C=CC(=C4)OS(=O)(=O)N
|
|||
| InChI |
1S/C18H23NO4S/c1-18-9-8-14-13-5-3-12(23-24(19,21)22)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16H,2,4,6-9H2,1H3,(H2,19,21,22)/t14-,15-,16+,18+/m1/s1
|
|||
| InChIKey |
RVKFQAJIXCZXQY-CBZIJGRNSA-N
|
|||
| CAS Number |
CAS 148672-09-7
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Carbonic anhydrase II (CA-II) | Target Info | Inhibitor | [2] |
| Steryl-sulfatase (STS) | Target Info | Inhibitor | [1] | |
| KEGG Pathway | Nitrogen metabolism | |||
| Proximal tubule bicarbonate reclamation | ||||
| Collecting duct acid secretion | ||||
| Gastric acid secretion | ||||
| Pancreatic secretion | ||||
| Bile secretion | ||||
| Steroid hormone biosynthesis | ||||
| NetPath Pathway | IL4 Signaling Pathway | |||
| EGFR1 Signaling Pathway | ||||
| Pathwhiz Pathway | Androgen and Estrogen Metabolism | |||
| Reactome | Erythrocytes take up carbon dioxide and release oxygen | |||
| Erythrocytes take up oxygen and release carbon dioxide | ||||
| Reversible hydration of carbon dioxide | ||||
| Glycosphingolipid metabolism | ||||
| WikiPathways | Reversible Hydration of Carbon Dioxide | |||
| Uptake of Carbon Dioxide and Release of Oxygen by Erythrocytes | ||||
| Uptake of Oxygen and Release of Carbon Dioxide by Erythrocytes | ||||
| Estrogen metabolism | ||||
| Vitamin D Receptor Pathway | ||||
| Sphingolipid metabolism | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Thiosemicarbazones of formyl benzoic acids as novel potent inhibitors of estrone sulfatase. J Med Chem. 2007 Jul 26;50(15):3661-6. | |||
| REF 2 | 2-substituted estradiol bis-sulfamates, multitargeted antitumor agents: synthesis, in vitro SAR, protein crystallography, and in vivo activity. J Med Chem. 2006 Dec 28;49(26):7683-96. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

