Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0RA9E
|
|||
| Former ID |
DNAP001401
|
|||
| Drug Name |
Ramosetron
|
|||
| Synonyms |
132036-88-5; UNII-7ZRO0SC54Y; Ramosetron [INN]; 7ZRO0SC54Y; CHEMBL1643895; Ramosetron (INN); (1-methylindol-3-yl)-[(5R)-4,5,6,7-tetrahydro-3H-benzimidazol-5-yl]methanone; Nor-YM 060; SCHEMBL16701; GTPL2301; DTXSID0043842; NTHPAPBPFQJABD-LLVKDONJSA-N; MolPort-019-991-383; CHEBI:135156; ZINC5116719; AC1L3355; BDBM50334454; 8235AH; AKOS015896003; SB19072; DB09290; SC-92398; AJ-53160; LS-187182; TL8000762; R-146; FT-0651831; D08466; A806353; (-)-(R)-1-Methylindol-3-yl-4,5,6,7-tetrahydro-5-benzimidazolyl ketone; Nasea (TN); YM060
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Nausea and vomiting [ICD-11: MD90; ICD-10: R11] | Approved | [1], [2] | |
| Irritable bowel syndrome [ICD-11: DD91.0; ICD-10: K55-K64, K58; ICD-9: 564.1, 787.91] | Phase 4 | [3] | ||
| Structure |
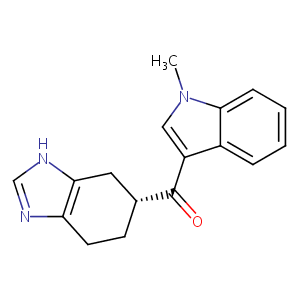 |
Download2D MOL |
||
| Formula |
C17H17N3O
|
|||
| Canonical SMILES |
CN1C=C(C2=CC=CC=C21)C(=O)C3CCC4=C(C3)NC=N4
|
|||
| InChI |
1S/C17H17N3O/c1-20-9-13(12-4-2-3-5-16(12)20)17(21)11-6-7-14-15(8-11)19-10-18-14/h2-5,9-11H,6-8H2,1H3,(H,18,19)/t11-/m1/s1
|
|||
| InChIKey |
NTHPAPBPFQJABD-LLVKDONJSA-N
|
|||
| CAS Number |
CAS 132036-88-5
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
14824418, 14848693, 44436913, 49830772, 50070718, 50112691, 71879502, 79053930, 96025152, 104380246, 124974479, 125084556, 128751655, 131297527, 135141836, 135650885, 137446772, 140430812, 152035422, 172659137, 179149824, 184546127, 198983023, 223562331, 224977577, 226406853, 241031501, 251912291, 251916599
|
|||
| ChEBI ID |
CHEBI:135156
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | 5-HT 3 receptor (5HT3R) | Target Info | Modulator | [4] |
| 5-HT receptor (5HTR) | Target Info | Modulator | [5] | |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2301). | |||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 3 | ClinicalTrials.gov (NCT01225237) A Study to Evaluate Efficacy of Ramosetron on Diarrhea-predominant Irritable Bowel Syndrome (IBS) in Male Patients. U.S. National Institutes of Health. | |||
| REF 4 | Inhibitory effect of YM060 on 5-HT3 receptor-mediated depolarization in colonic myenteric neurons of the guinea pig. Eur J Pharmacol. 1995 Sep 5;283(1-3):107-12. | |||
| REF 5 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

