Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0RD6G
|
|||
| Former ID |
DNC007966
|
|||
| Drug Name |
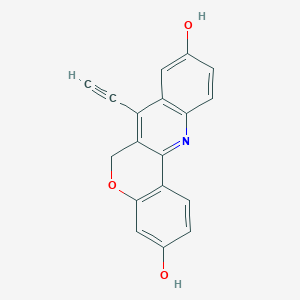
7-ethynyl-6H-chromeno[4,3-b]quinoline-3,9-diol
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
 |
Download2D MOL |
||
| Formula |
C18H11NO3
|
|||
| Canonical SMILES |
C#CC1=C2COC3=C(C2=NC4=C1C=C(C=C4)O)C=CC(=C3)O
|
|||
| InChI |
1S/C18H11NO3/c1-2-12-14-7-10(20)4-6-16(14)19-18-13-5-3-11(21)8-17(13)22-9-15(12)18/h1,3-8,20-21H,9H2
|
|||
| InChIKey |
NFDYBNVVFHAKIV-UHFFFAOYSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Estrogen-related receptor-alpha (ESRRA) | Target Info | Inhibitor | [1] |
| Reactome | PPARA activates gene expression | |||
| Transcriptional activation of mitochondrial biogenesis | ||||
| Nuclear Receptor transcription pathway | ||||
| WikiPathways | Mitochondrial Gene Expression | |||
| Regulation of Lipid Metabolism by Peroxisome proliferator-activated receptor alpha (PPARalpha) | ||||
| Nuclear Receptors | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ERbeta ligands. Part 6: 6H-Chromeno[4,3-b]quinolines as a new series of estrogen receptor beta-selective ligands. Bioorg Med Chem Lett. 2007 Jul 15;17(14):4053-6. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

