Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0S5LT
|
|||
| Former ID |
DNC004647
|
|||
| Drug Name |
Deoxynojirimycin
|
|||
| Synonyms |
1-DEOXYNOJIRIMYCIN; 19130-96-2; DUVOGLUSTAT; deoxynojirimycin; Moranoline; (2R,3R,4R,5S)-2-(hydroxymethyl)piperidine-3,4,5-triol; 1,5-Deoxy-1,5-imino-D-mannitol; Moranolin; UNII-FZ56898FLE; D-1-deoxynojirimycin; 1,5-Dideoxy-1,5-imino-D-glucitol; CHEMBL307429; C6H13NO4; FZ56898FLE; CHEBI:44369; 1-Deoxy-Nojirimycin; 5-Amino-1,5-dideoxy-D-glucopyranose; DNJ; AK151410; 3,4,5-Piperidinetriol, 2-(hydroxymethyl)-, (2R,3R,4R,5S)-; 3,4,5-Piperidinetriol, 2-(hydroxymethyl)-, (2R-(2alpha,3beta,4alpha,5beta))-
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Pompe disease [ICD-11: 5C51.3; ICD-10: E74.0] | Phase 3 | [1] | |
| Structure |
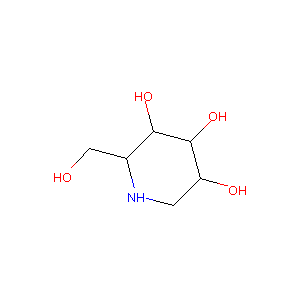 |
Download2D MOL |
||
| Formula |
C6H13NO4
|
|||
| Canonical SMILES |
C1C(C(C(C(N1)CO)O)O)O
|
|||
| InChI |
1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4+,5-,6-/m1/s1
|
|||
| InChIKey |
LXBIFEVIBLOUGU-JGWLITMVSA-N
|
|||
| CAS Number |
CAS 19130-96-2
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:44369
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Alpha-glucosidase (GLA) | Target Info | Inhibitor | [2] |
| Intestinal maltase-glucoamylase (MGAM) | Target Info | Inhibitor | [2] | |
| Lysosomal alpha-glucosidase (GAA) | Target Info | Inhibitor | [3] | |
| KEGG Pathway | Galactose metabolism | |||
| Starch and sucrose metabolism | ||||
| Metabolic pathways | ||||
| Carbohydrate digestion and absorption | ||||
| Lysosome | ||||
| Pathwhiz Pathway | Starch and Sucrose Metabolism | |||
| Galactose Metabolism | ||||
| Pathway Interaction Database | Notch-mediated HES/HEY network | |||
| WikiPathways | Metabolism of carbohydrates | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | The pharmacological chaperone 1-deoxynojirimycin increases the activity and lysosomal trafficking of multiple mutant forms of acid alpha-glucosidase. Hum Mutat. 2009 Dec;30(12):1683-92. | |||
| REF 2 | Nitrogen-in-the-ring pyranoses and furanoses: structural basis of inhibition of mammalian glycosidases. J Med Chem. 1994 Oct 28;37(22):3701-6. | |||
| REF 3 | In vitro inhibition of glycogen-degrading enzymes and glycosidases by six-membered sugar mimics and their evaluation in cell cultures. Bioorg Med Chem. 2008 Aug 1;16(15):7330-6. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

