Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0SD9Z
|
|||
| Drug Name |
TRC-253
|
|||
| Synonyms |
OUEHJEYKNYQVRC-UHFFFAOYSA-N; TRC253; SCHEMBL19128768; EX-A1808; 2110426-27-0; 5-(8-oxo-5-(6-(piperidin-4-yloxy)pyridin-3-yl)-6-thioxo-5,7-diazaspiro[3.4]octan-7-yl)-3-(trifluoromethyl)picolinonitrile
Click to Show/Hide
|
|||
| Indication | Prostate cancer [ICD-11: 2C82.0; ICD-9: 185] | Phase 1/2 | [1] | |
| Company |
Janssen Research & Development Raritan, NJTRACON PharmaceuticalsSan Diego, CA
|
|||
| Structure |
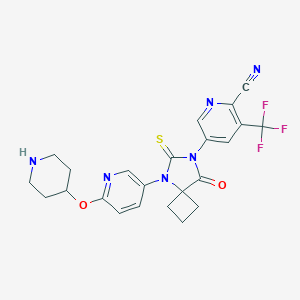 |
Download2D MOL |
||
| Formula |
C23H21F3N6O2S
|
|||
| Canonical SMILES |
C1CC2(C1)C(=O)N(C(=S)N2C3=CN=C(C=C3)OC4CCNCC4)C5=CC(=C(N=C5)C#N)C(F)(F)F
|
|||
| InChI |
1S/C23H21F3N6O2S/c24-23(25,26)17-10-15(13-29-18(17)11-27)31-20(33)22(6-1-7-22)32(21(31)35)14-2-3-19(30-12-14)34-16-4-8-28-9-5-16/h2-3,10,12-13,16,28H,1,4-9H2
|
|||
| InChIKey |
OUEHJEYKNYQVRC-UHFFFAOYSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Androgen receptor messenger RNA (AR mRNA) | Target Info | Antagonist | [2] |
| KEGG Pathway | Oocyte meiosis | |||
| Pathways in cancer | ||||
| Prostate cancer | ||||
| NetPath Pathway | EGFR1 Signaling Pathway | |||
| AndrogenReceptor Signaling Pathway | ||||
| FSH Signaling Pathway | ||||
| Pathway Interaction Database | Regulation of nuclear SMAD2/3 signaling | |||
| Coregulation of Androgen receptor activity | ||||
| Regulation of Androgen receptor activity | ||||
| Nongenotropic Androgen signaling | ||||
| Regulation of nuclear beta catenin signaling and target gene transcription | ||||
| FOXA1 transcription factor network | ||||
| Notch-mediated HES/HEY network | ||||
| Reactome | Nuclear Receptor transcription pathway | |||
| Activated PKN1 stimulates transcription of AR (androgen receptor) regulated genes KLK2 and KLK3 | ||||
| WikiPathways | SIDS Susceptibility Pathways | |||
| Integrated Pancreatic Cancer Pathway | ||||
| Prostate Cancer | ||||
| Integrated Breast Cancer Pathway | ||||
| Nuclear Receptors | ||||
| Androgen receptor signaling pathway | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT02987829) Phase 1/2A Study of TRC253, an Androgen Receptor Antagonist, in Metastatic Castration-resistant Prostate Cancer Patients. U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

