Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0SV8E
|
|||
| Drug Name |
Methotrexate
|
|||
| Synonyms |
methotrexate; 1959/5/2; Rheumatrex; Amethopterin; Metatrexan; Hdmtx; Abitrexate; Mexate; Methylaminopterinum; Methotrexatum; Antifolan; Metotrexato; Methylaminopterin; MTX; (S)-2-(4-(((2,4-Diaminopteridin-6-yl)methyl)(methyl)amino)benzamido)pentanedioic acid; Methotrexat; Amethopterine; Maxtrex; Rasuvo; L-Amethopterin; A-Methopterin; A-Methpterin; Amethopterin L-; Folex-Pfs; Methotrexat-Ebewe; N-Bismethylpteroylglutamic acid; Methotrexate, L-; Metotressato [DCIT]; Methotextrate; Mexate-Aq; [3H]methotrexate
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | leukaemia [ICD-11: 2A60-2B33; ICD-9: 208.9] | Approved | [1] | |
| Proliferative vitreoretinopathy [ICD-11: 9B78.2] | Phase 3 | [2] | ||
| Solid tumour/cancer [ICD-11: 2A00-2F9Z; ICD-10: C76-C80; ICD-9: 140-229] | Phase 3 | [1] | ||
| Rheumatoid arthritis [ICD-11: FA20] | Phase 1 | [3] | ||
| Prostate cancer [ICD-11: 2C82.0; ICD-10: C61; ICD-9: 185] | Investigative | [4] | ||
| Structure |
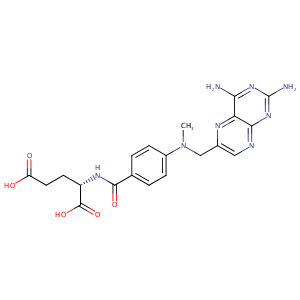 |
Download2D MOL
|
||
| Formula |
C20H22N8O5
|
|||
| Canonical SMILES |
CN(CC1=CN=C2C(=N1)C(=NC(=N2)N)N)C3=CC=C(C=C3)C(=O)NC(CCC(=O)O)C(=O)O
|
|||
| InChI |
1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1
|
|||
| InChIKey |
FBOZXECLQNJBKD-ZDUSSCGKSA-N
|
|||
| CAS Number |
CAS 59-05-2
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:44185
|
|||
| ADReCS Drug ID | BADD_D01418 | |||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Metabolism of Drug Affected by Studied Microbe(s) | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Gut microbiota | ||||
| Studied Microbe: Gut microbiota unspecific | [5], [6] | |||
| Metabolic Effect | Decrease toxicity; Decrease activity | |||
| Description | Methotrexate can be metabolized by gut microbiota, which results in the decrease of the drug's toxicity and activity. | |||
| The Abundace of Studied Microbe(s) Regulated by Drug | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Bacteroidales | ||||
|
Studied Microbe: Bacteroides
Show/Hide Hierarchy
|
[7] | |||
| Hierarchy | ||||
| Abundance Change | Increase | |||
| Experimental Species | Wistar Unilever outbred rat | Experimental Sample | Faeces | |
| Disease or Condition | Gastrointestinal mucositis | |||
| Description | The abundance of Bacteroides was increased by Methotrexate. | |||
|
Studied Microbe: Bacteroides fragilis
Show/Hide Hierarchy
|
[8], [9] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experimental Species | BALB/c mice | Experimental Sample | Faeces | |
| Disease or Condition | Acute lymphocytic leukemia | |||
| Description | The abundance of Bacteroides fragilis was decreased by Methotrexate (p?<?0.01). | |||
|
Studied Microbe: Bacteroides uniformis
Show/Hide Hierarchy
|
[10] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Bacteroides uniformis was decreased by Methotrexate (adjusted p-values: 7.64E-07). | |||
|
Studied Microbe: Bacteroides vulgatus
Show/Hide Hierarchy
|
[10] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Bacteroides vulgatus was decreased by Methotrexate (adjusted p-values: 8.82E-05). | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Erysipelotrichales | ||||
|
Studied Microbe: Erysipelatoclostridium ramosum
Show/Hide Hierarchy
|
[10] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Erysipelatoclostridium ramosum was decreased by Methotrexate (adjusted p-values: 1.34E-05). | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Eubacteriales | ||||
|
Studied Microbe: Clostridium perfringens
Show/Hide Hierarchy
|
[10] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Clostridium perfringens was decreased by Methotrexate (adjusted p-values: 2.54E-04). | |||
|
Studied Microbe: Enterocloster bolteae
Show/Hide Hierarchy
|
[10] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Enterocloster bolteae was decreased by Methotrexate (adjusted p-values: 1.17E-04). | |||
|
Studied Microbe: Eubacterium rectale
Show/Hide Hierarchy
|
[10] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Eubacterium rectale was decreased by Methotrexate (adjusted p-values: 1.93E-03). | |||
|
Studied Microbe: Lachnospiraceae
Show/Hide Hierarchy
|
[8], [9] | |||
| Hierarchy | ||||
| Abundance Change | Increase | |||
| Experimental Species | BALB/c mice | Experimental Sample | Faeces | |
| Disease or Condition | Acute lymphocytic leukemia | |||
| Description | The abundance of Lachnospiraceae was increased by Methotrexate. | |||
|
Studied Microbe: Roseburia hominis
Show/Hide Hierarchy
|
[10] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Roseburia hominis was decreased by Methotrexate (adjusted p-values: 6.37E-03). | |||
|
Studied Microbe: Ruminococcus gnavus
Show/Hide Hierarchy
|
[10] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Ruminococcus gnavus was decreased by Methotrexate (adjusted p-values: 5.15E-03). | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Lactobacillales | ||||
|
Studied Microbe: Lactobacillus paracasei
Show/Hide Hierarchy
|
[10] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Lactobacillus paracasei was decreased by Methotrexate (adjusted p-values: 6.45E-07). | |||
|
Studied Microbe: Streptococcus
Show/Hide Hierarchy
|
[7] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experimental Species | Wistar Unilever outbred rat | Experimental Sample | Faeces | |
| Disease or Condition | Gastrointestinal mucositis | |||
| Description | The abundance of Streptococcus was decreased by Methotrexate. | |||
|
Studied Microbe: Streptococcus parasanguinis
Show/Hide Hierarchy
|
[10] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Streptococcus parasanguinis was decreased by Methotrexate (adjusted p-values: 5.51E-07). | |||
|
Studied Microbe: Streptococcus salivarius
Show/Hide Hierarchy
|
[10] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Streptococcus salivarius was decreased by Methotrexate (adjusted p-values: 5.77E-07). | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Veillonellales | ||||
|
Studied Microbe: Veillonella parvula
Show/Hide Hierarchy
|
[10] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Veillonella parvula was decreased by Methotrexate (adjusted p-values: 5.04E-06). | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Gut microbiota | ||||
| Studied Microbe: Anaerobes | [7] | |||
| Abundance Change | Decrease | |||
| Experimental Species | Wistar Unilever outbred rat | Experimental Sample | Faeces | |
| Disease or Condition | Gastrointestinal mucositis | |||
| Description | The abundance of Anaerobes was decreased by Methotrexate. | |||
| Drug Resistance Mutation (DRM) | Top | |||
|---|---|---|---|---|
| DRM | DRM Info | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Proton-coupled folate transporter (SLC46A1) | Target Info | Modulator | [11] |
| Solute carrier family 19 member 1 (SLC19A1) | Target Info | Modulator | [12] | |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Pain reduction with oral methotrexate in knee osteoarthritis, a pragmatic phase iii trial of treatment effectiveness (PROMOTE): study protocol for ... Trials. 2015 Mar 4;16:77. | |||
| REF 2 | ClinicalTrials.gov (NCT04136366) The GUARD Trial - Part 1: A Phase 3 Clinical Trial for Prevention of Proliferative Vitreoretinopathy. U.S. National Institutes of Health. | |||
| REF 3 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800034238) | |||
| REF 4 | The ChEMBL database in 2017. Nucleic Acids Res. 2017 Jan 4;45(D1):D945-D954. | |||
| REF 5 | Pharmacomicrobiomics: exploiting the drug-microbiota interactions in anticancer therapies. Microbiome. 2018 May 22;6(1):92. | |||
| REF 6 | The microbial pharmacists within us: a metagenomic view of xenobiotic metabolism. Nat Rev Microbiol. 2016 Apr;14(5):273-87. | |||
| REF 7 | Substantial decreases in the number and diversity of microbiota during chemotherapy-induced gastrointestinal mucositis in a rat model. Support Care Cancer. 2015 Jun;23(6):1513-22. | |||
| REF 8 | Induction and Amelioration of Methotrexate-Induced Gastrointestinal Toxicity are Related to Immune Response and Gut Microbiota. EBioMedicine. 2018 Jul;33:122-133. | |||
| REF 9 | Emerging Insights on the Interaction Between Anticancer and Immunosuppressant Drugs and Intestinal Microbiota in Pediatric Patients. Clin Transl Sci. 2020 Mar;13(2):238-259. | |||
| REF 10 | Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018 Mar 29;555(7698):623-628. | |||
| REF 11 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 1213). | |||
| REF 12 | Increased activity of a novel low pH folate transporter associated with lipophilic antifolate resistance in chinese hamster ovary cells. J Biol Chem. 1998 Apr 3;273(14):8106-11. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

