Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0T5AW
|
|||
| Former ID |
DIB004852
|
|||
| Drug Name |
Desoxypeganine
|
|||
| Synonyms |
Alcoholism therapy, HF Arzneimittelforschung; Desoxypeganine, HF Arzneimittelforschung
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Alcohol dependence [ICD-11: 6C40.2; ICD-10: F10.2; ICD-9: 303] | Phase 1 | [1] | |
| Company |
HF Arzneimittelforschung GmbH & Co KG
|
|||
| Structure |
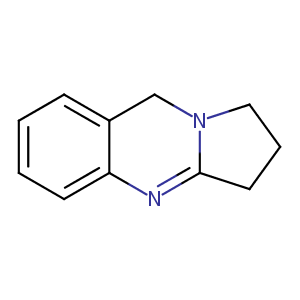 |
Download2D MOL |
||
| Formula |
C11H12N2
|
|||
| Canonical SMILES |
C1CC2=NC3=CC=CC=C3CN2C1
|
|||
| InChI |
1S/C11H12N2/c1-2-5-10-9(4-1)8-13-7-3-6-11(13)12-10/h1-2,4-5H,3,6-8H2
|
|||
| InChIKey |
WUFQLZTXIWKION-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 495-59-0
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:4428
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Phase I clinical trial with desoxypeganine, a new cholinesterase and selective MAO-A inhibitor: tolerance and pharmacokinetics study of escalating single oral doses. Methods Find Exp Clin Pharmacol. 2008 Mar;30(2):141-7. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

